Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural diversity of alkylzinc complexes with pyrrole-based N,O-ligands: from molecular complexes to coordination polymers†
Zbigniew
Wróbel
a,
Iwona
Justyniak
a,
Izabela
Dranka
b and
Janusz
Lewiński
*ab
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
bDepartment of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: lewin@ch.pw.edu.pl
First published on 29th March 2016
Abstract
The equimolar reaction of R2Zn (where R = Et or tBu) with pyrrole-based N,O-proligands afforded a series of alkylzinc compounds with a variety of intriguing structures including a hexanuclear macrocyclic complex and 1D coordination polymers with versatile intramolecular or intermolecular bonding modes.
The variety of chemistries displayed by organozinc complexes supported by ligand systems that incorporate a pyrrolide anion and terminal N′-donor sites is well documented. The combination of the pyrrolyl skeleton and N-donor sites renders N,N′- and N′,N,N′-multifunctional ligands with versatile intramolecular or intermolecular bonding modes to zinc centers.1–3 It is also pertinent to note that multidentate ligands with pyrrolic units in combination with Zn(II) ions have appeared as an interesting building block for the supramolecular architecture through self-assembly.4,5 Moreover, alkylzinc complexes with these versatile ligands show remarkable reactivity in a variety of metal-mediated transformations.2a,b,6,7 Our previous studies show that N,N′-pyrroles (H-L1, Scheme 1) are versatile supporting proligands. For example, the controlled oxygenation of [RZn(N,N′)] complexes (where N,N′ = 2,2′-(1′-pyrrolinyl)-pyrrole) provided a novel zinc alkyl peroxide or zinc oxo-encapsulated cluster, the formation of which was mediated by the nature of zinc-bonded alkyl substituents.6b Strikingly, in the case of a [EtZn(N′,N,N′)] complex (where N′,N,N′ = deprotonated 2,5-bis[(2,6-diisopropylphenyl)-aldimino]-pyrrole) the oxygenation of the Et–Zn subunit led to the formation of zinc acetate species.6c This particular ligand set is also able to promote the catalytic activity of both zinc alkoxides in the ring-opening polymerization of cyclic esters,8 or zinc alkylperoxides in the asymmetric epoxidations of enones.9 Surprisingly, the related ligand systems incorporating a pyrrolide anion and a terminal carbonyl O-donor site still remain poorly explored in the field of organozinc chemistry. As a part of our ongoing interest in the development of new reaction systems based on zinc complexes supported by multidentate pyrrole-based ligands, herein we have focused on the versatile bonding modes of monoanionic pyrrolate ligands derived from 1H-pyrrole-2-carboxylate (H–PyrC(O)OMe) and 1H-pyrrole-2-carboxaldehyde (H–PyrC(O)H) as model proligands (H-L2, Scheme 1).
The equimolar reaction of R2Zn (where R = Et or tBu) with 1 equiv. of methyl 1H-pyrrole-2-carboxylate (H–PyrC(O)OMe) in a non-coordinating solvent at −78 °C afforded the alkylzinc complexes of general formula [RZn(PyrC(O)OMe)]n, where R = Et (1n) and tBu (2), respectively (Scheme 2). The single crystals of 1n suitable for the X-ray diffraction studies were isolated from a toluene solution. Despite numerous attempts, the isolation of single crystals of 2 suitable for X-ray diffraction studies was unsuccessful due to its good solubility, thus complex 2 was characterized spectroscopically (for details see the ESI†).
Complex 1n crystallized as colorless needles in the P21/m space group. The monomeric basic unit of 1n consists of the three-coordinated zinc center supported by one pyrrolide PyrC(O)OMe ligand (Fig. 1a). The coordination sphere of the zinc atom is completed with the ethyl group. The central five-membered ring formed by the N,O-ligand and the metal center is planar (the torsion angle between C6–C7–O1–Zn1 is 0.00° with the ethyl group located in the same plane). The Zn1–N1pyrrole and Zn1–O1carbonyl bond lengths are 1.930(3) Å and 2.185(3) Å, respectively. The crystal structure analysis revealed that the molecules of 1 form a 1D coordination polymer from the alternating molecular units. The adjacent molecules of 1 are connected by a network of intermolecular Zn⋯π interactions between the coordinatively unsaturated metal centers and the C(4) carbon atoms of the pyrrolide ring (with the Zn1⋯π(C4′) and Zn1⋯π(C4′′) distances of 3.119(2) Å, dotted lines in Fig. 1b). Similar Zn⋯π interactions were observed previously for a dimeric [tBuZn(PyrPri)]2 complex supported by a bifunctional N,N-pyrrolylaldiminato ligand.1e,7 We note that in contrast to 1, the related low-alkyl zinc complexes incorporating pyrrolylaldiminato ligands were unstable and the formation of bis(pyrrolylaldiminato) compounds was only observed.1a,b,f,g,3c
To understand more in depth the factors which determine the structure and stability of alkylzinc complexes supported by N,O-pyrrole based monoanionic ligands, we investigated the reactivity of 1H-pyrrole-2-carboxaldehyde (H–PyrC(O)H) towards R2Zn species (where R = Et or tBu). The equimolar reaction between H–PyrC(O)H and R2Zn at −78 °C afforded two alkylzinc complexes of the general formula [RZn(PyrC(O)H)]n, where R = Et (3n) and tBu (46), respectively (Scheme 2). In both cases colorless needle-like crystals suitable for X-ray diffraction measurements were isolated from a toluene solution at room temperature. Compound 3n crystallizes in the monoclinic space group P21/c as a 1D zig-zag coordination polymer (Fig. 2). The molecular unit 3 consists of a three-coordinate ethylzinc species supported by a chelating monoanionic N,O-pyrrole-2-carboxaldehyde ligand. The carbonyl oxygen atom O1 bridges the adjacent units of 3 with a μ2-O mode and fulfills the coordination sphere of the metal center. Each [EtZn(PyrC(O)H)] unit is nearly planar (the torsion angle between C6–C7–O1–Zn1 is 0.9(4)°). The analysis of Zn1–O bond lengths clearly indicates the stronger coordination of the zinc center to the O1′ carbonyl oxygen atom from the adjacent unit when compared to the O1 atom (the Zn–Ocarbonyl bond distances fall in the range of 2.091–2.189 Å, and the C–O distance is 1.292(5) Å). The supramolecular structure of 3n consists of repeating monomeric units [EtZn(PyrC(O)H)] rotated relative to one another by ca. 72°.
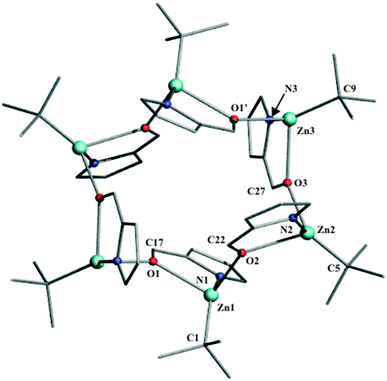
Compound 46 crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group from a toluene solution at room temperature.‡ The single-crystal X-ray diffraction analysis demonstrated that in the solid state complex 46 exists as a hexanuclear macrocyclic cluster [tBuZn(PyrC(O)H)]6. The molecular structure of 46 consists of six discrete monomeric units bridged by the oxygen atom of the aldehyde group with a μ2-O mode (Fig. 3). The geometry of the zinc atoms in 46 is a distorted tetrahedral with angles in the range of 80.2° to 142.3°. The bond distance between the zinc center and the oxygen atoms is between 2.084 and 2.240 Å, while the Zn–Npyrrole distance varies from 1.976 to 1.996 Å (the average C–O distance is 1.280 Å). Similarly as observed in 3n, the analysis of Zn–Ocarbonyl bond lengths in 46 confirmed the stronger binding affinity of the Zn atom to the carbonyl oxygen atom from the adjacent [tBuZn(PyrC(O)H)] unit. We note that the average Zn–Ocarbonyl bond distances in 3n and 46 are significantly longer than that observed in dinuclear zinc complexes incorporating the bridging μ-O-enolate ligand (the corresponding Zn–O bond lengths fall around 2.0 Å)10 as well as in a dimeric ethylzinc complex incorporating the μ-O,N-enolate chelating ligand, [EtZn(μ-O(Me)
space group from a toluene solution at room temperature.‡ The single-crystal X-ray diffraction analysis demonstrated that in the solid state complex 46 exists as a hexanuclear macrocyclic cluster [tBuZn(PyrC(O)H)]6. The molecular structure of 46 consists of six discrete monomeric units bridged by the oxygen atom of the aldehyde group with a μ2-O mode (Fig. 3). The geometry of the zinc atoms in 46 is a distorted tetrahedral with angles in the range of 80.2° to 142.3°. The bond distance between the zinc center and the oxygen atoms is between 2.084 and 2.240 Å, while the Zn–Npyrrole distance varies from 1.976 to 1.996 Å (the average C–O distance is 1.280 Å). Similarly as observed in 3n, the analysis of Zn–Ocarbonyl bond lengths in 46 confirmed the stronger binding affinity of the Zn atom to the carbonyl oxygen atom from the adjacent [tBuZn(PyrC(O)H)] unit. We note that the average Zn–Ocarbonyl bond distances in 3n and 46 are significantly longer than that observed in dinuclear zinc complexes incorporating the bridging μ-O-enolate ligand (the corresponding Zn–O bond lengths fall around 2.0 Å)10 as well as in a dimeric ethylzinc complex incorporating the μ-O,N-enolate chelating ligand, [EtZn(μ-O(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (H)CN(Et)tBu)]2 (average Zn–O bond distance is 2.067 Å).11 Moreover, the 12-membered ring of 46 comprises six zinc atoms that are linked via Ocarbonyl atoms in the μ-O bridging fashion (Fig. 3). The Zn6(μ-O)6 central ring is non-planar around which the pyrrole ligands self-organize in an alternating “up and down” manner. All of the tBu groups are directed outside of this macrocyclic ring. Thus, the structural analyses of 3n and 46 revealed that the presence of a more bulky tBu group favors the formation of a hexanuclear macrocycle. Notably, in 3n and 46 the coordination mode of pyrrole ligands through the Ocarbonyl atom is unusual compared with the reported pyrrole-based complexes, which commonly appear as dimeric species joined by π or δ interactions between the metal center and the nitrogen atom from the pyrrole ring.
(H)CN(Et)tBu)]2 (average Zn–O bond distance is 2.067 Å).11 Moreover, the 12-membered ring of 46 comprises six zinc atoms that are linked via Ocarbonyl atoms in the μ-O bridging fashion (Fig. 3). The Zn6(μ-O)6 central ring is non-planar around which the pyrrole ligands self-organize in an alternating “up and down” manner. All of the tBu groups are directed outside of this macrocyclic ring. Thus, the structural analyses of 3n and 46 revealed that the presence of a more bulky tBu group favors the formation of a hexanuclear macrocycle. Notably, in 3n and 46 the coordination mode of pyrrole ligands through the Ocarbonyl atom is unusual compared with the reported pyrrole-based complexes, which commonly appear as dimeric species joined by π or δ interactions between the metal center and the nitrogen atom from the pyrrole ring.
Conclusions
In conclusion, we demonstrated the diversity in bonding modes of pyrrole-based N,O-bifunctional monoanionic ligands in the solid state which resulted in the formation of a series of novel alkylzinc complexes. We isolated and characterized for the first time the non-covalent coordination polymers connected through Zn–π interactions. Moreover, we demonstrated a new type of connectivity in the alkylzinc pyrrole based complexes, through the Zn–Ocarbonyl interactions. These new types of interactions shed a new light on pyrrole-based complexes and could provide useful tools in the field of molecular engineering. Further studies on the factors controlling the structure and reactivity of the alkylzinc complexes supported by multifunctional N,O-pyrrole ligands are in progress.Acknowledgements
The authors would like to acknowledge the National Science Centre, Grant Maestro DEC-2012/04/A/ST5/00595, for financial support.Notes and references
- For zinc complexes with pyrrole-based N,N′-ligands, see: (a) S. Qiao, W.-A. Ma and Z.-X. Wang, J. Organomet. Chem., 2011, 696, 2746 CrossRef CAS; (b) H. Wang, Y. Zeng, J. S. Ma, H. Fu, J. Yao, A. I. Mikhaleva and B. A. Trofimov, Chem. Commun., 2009, 5457 RSC; (c) C. S. B. Gomes, P. T. Gomes, M. T. Duarte, R. E. D. Paolo, A. L. Macanita and M. J. Calhorda, Inorg. Chem., 2009, 48, 11176 CrossRef CAS PubMed; (d) Y. Wang, H. Fu, F. Shen, X. Sheng, A. Peng, Z. Gu, H. Ma, J. S. Ma and J. Yao, Inorg. Chem., 2007, 46, 3548 CrossRef CAS PubMed; (e) J. Lewiński, M. Dranka, I. Kraszewska, W. Śliwinski and I. Justyniak, Chem. Commun., 2005, 4935 RSC; (f) J. J. Klappa, S. A. Geers, S. J. Schmidtke, L. A. MacManus-Spencer and K. McNeill, Dalton Trans., 2004, 883 RSC; (g) H. J. Hao, S. Bhandari, Y. Q. Ding, H. W. Roesky, J. Magull, H. G. Schmidt, M. Noltemeyer and C. M. Cui, Eur. J. Inorg. Chem., 2002, 5, 1060 CrossRef.
- For zinc complexes with pyrrole-based N′,N,N′-ligands, see: (a) K.-M. Chien, T.-C. Hu, C.-H. Lin, Y.-C. Lo, T.-Y. Lee and J.-H. Huang, J. Organomet. Chem., 2015, 779, 39 CrossRef CAS; (b) M.-C. Wu, T.-C. Hu, Y.-C. Lo, T.-Y. Lee, C.-H. Lin, W.-H. Lu, C.-C. Lin, A. Datta and J.-H. Huang, J. Organomet. Chem., 2015, 791, 141 CrossRef CAS; (c) Q. Liu, Z. Guo, H. Han, H. Tong and X. We, Polyhedron, 2015, 85, 15 CrossRef CAS; (d) H. V. Babu and K. Muralidharan, Dalton Trans., 2013, 42, 1238 RSC.
- For selected zinc complexes with pyrrole-based macrocyclic ligands, see: (a) T. Cadenbach, J. R. Pankhurst, T. A. Hofmann, M. Curcio, P. L. Arnold and J. B. Love, Organometallics, 2015, 34, 2608 CrossRef CAS; (b) A. M. J. Devoille, P. Richardson, N. L. Bill, J. L. Sessler and J. B. Love, Inorg. Chem., 2011, 50, 3116 CrossRef CAS PubMed; (c) S. D. Reid, C. Wilson, C. I. De Matteis and J. B. Love, Eur. J. Inorg. Chem., 2007, 5286 CrossRef CAS; (d) S. D. Reid, A. J. Blake, C. Wilson and J. B. Love, Inorg. Chem., 2006, 45, 636 CrossRef CAS PubMed.
- (a) G. Givaja, A. J. Blake, C. Wilson, M. Schroder and J. B. Love, Chem. Commun., 2005, 4423 RSC; (b) Z. Wu, Q. Chen, S. Xiong, B. Xin, Z. Zhao, L. Jiang and J. S. Ma, Angew. Chem., Int. Ed., 2003, 42, 3271 CrossRef CAS PubMed.
- (a) S. A. Baudron, H. Ruffin and M. W. Hosseini, Chem. Commun., 2015, 51, 5906 RSC and references therein; (b) S. D. Reid, A. J. Blacke, C. Wilson and J. B. Love, Inorg. Chem., 2006, 45, 636 CrossRef CAS PubMed.
- (a) N. Komine, R. W. Buell, C.-H. Chen, A. K. Hui, M. Pink and K. G. Caulton, Inorg. Chem., 2014, 53, 1361 CrossRef CAS PubMed; (b) J. Lewiński, K. Suwała, T. Kaczorowski, M. Gałęzowski, D. T. Gryko, I. Justyniak and J. Lipkowski, Chem. Commun., 2009, 215 RSC; (c) J. Lewiński, M. Kościelski, K. Suwała and I. Justyniak, Angew. Chem., Int. Ed., 2009, 48, 7017 CrossRef PubMed.
- B. Vidjayacoumar, D. J. H. Emslie, J. M. Blackwell, S. B. Clendenning and J. F. Britten, Chem. Mater., 2010, 22, 4854 CrossRef CAS.
- (a) C.-S. Hsiao, T.-Y. Wang, A. Datta, F.-X. Liao, C.-H. Hu, C.-H. Lin, J.-H. Huang and T.-Y. Lee, J. Organomet. Chem., 2012, 718, 82 CrossRef CAS; (b) W.-L. Kong and Z.-X. Wang, Dalton Trans., 2014, 43, 9126 RSC.
- (a) A. R. Keeri, I. Justyniak, J. Jurczak and J. Lewiński, Adv. Synth. Catal., 2016, 358, 864 CrossRef; (b) M. Kubisiak, K. Zelga, I. Justyniak, E. Tratkiewicz, T. Pietrzak, A. R. Keeri, Z. Ochal, L. Hartenstein, P. W. Roesky and J. Lewiński, Organometallics, 2013, 32, 5263 CrossRef CAS.
- M. L. Hlavinka and J. R. Hagadorn, Organometallics, 2006, 25, 3501 CrossRef CAS.
- M. R. P. van Vliet, G. van Koten, P. Buysingh, J. T. B. H. Jastrzebski and A. L. Speklb, Organometallics, 1987, 6, 537 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: All experimental details and characterization data. CCDC 1453366–1453368. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00674d |
| ‡ Crystal data for 1n; [C8H11NO2Zn]n: M = 218.55, crystal dimensions 0.30 × 0.15 × 0.08 mm3, monoclinic, space group P21/m (no. 11), a = 8.5170(6) Å, b = 6.2320(3) Å, c = 8.7800(6) Å, β = 112.358(3)°, U = 430.99(5) Å3, Z = 2, F(000) = 224, Dc = 1.684 g cm−3, T = 100(2) K, μ(Mo-Kα) = 2.804 mm−1, Nonius Kappa-CCD diffractometer, θmax = 27.60°, R1 = 0.0488, wR2 = 0.0899 for all data, R1 = 0.0398, wR2 = 0.0847 for 932 reflections with Io > 2σ(Io). The residual electron density = +0.45/−1.35 e Å−3. CCDC 1453366. Crystal data for 3n; [(C7H9NOZn)·0.5(C7H8]n: M = 234.59, crystal dimensions 0.28 × 0.14 × 0.10 mm3, monoclinic, space group P21/c (no. 14), a = 9.3370(9) Å, b = 5.4990(4) Å, c = 20.667(2) Å, β = 100.267(3)°, U = 1044.14(16) Å3, Z = 2, F(000) = 484, Dc = 1.492 g cm−3, T = 100(2) K, μ(Mo-Kα) = 2.314 mm−1, Nonius Kappa-CCD diffractometer, θmax = 27.482°, R1 = 0.0652, wR2 = 0.1064 for all data, R1 = 0.0508, wR2 = 0.1007 for 1880 reflections with Io > 2σ(Io). The residual electron density = +0.70/−0.61 e Å−3. CCDC 1453367. Crystal data for 46; C54H78N6O6Zn6: M = 1299.44, crystal dimensions 0.37 × 0.24 × 0.12 mm3, triclinic, space group P |
| This journal is © The Royal Society of Chemistry 2016 |