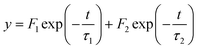Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emission behaviour of a series of bimetallic Cd(II)–Au(I) coordination polymers†
Akihiko
Yamagishi
a,
Takeshi
Kawasaki
a,
Kei
Hiruma
a,
Hisako
Sato
*b and
Takafumi
Kitazawa
*a
aDepartment of Chemistry, Toho University, Funabashi, Chiba, Japan
bGraduated School of Science and Engineering, Ehime University, Matsuyama, Ehime, Japan. E-mail: sato.hisako.my@ehime-u.ac.jp
First published on 23rd March 2016
Abstract
A series of bimetallic coordination polymers with the elemental composition of [CdIIL2][Au(CN)2]2, (L = 3-methylpyridine, 4-ethylpyridine, 3,5-lutidine and 3-fluoropyridine) were synthesized and their crystal structures were determined. In all of the investigated compounds, there existed a pair of Au–Au atoms whose interatomic distance was shorter than the sum of van der Waals radii (0.36 nm) as an indication of the aurophilic interaction. The compounds were emissive under the irradiation at 370 nm. The emission spectra recorded in the temperature range of 183–363 K were characterized by the vibronic structures with a peak spacing (Δν) of ca. 2000 cm−1. The value of Δν was close to the stretching vibration of the coordinated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (2150–2170 cm−1). It was postulated that C
N (2150–2170 cm−1). It was postulated that C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups participated in the emission processes through their vibronic coupling. In the case of L = 4-ethylpyridine, the lifetime of emission was measured in the same temperature range, leading to the conclusion that the activation energy of the non-radiative processes (ΔEa) was estimated to be 20 kJ mol−1.
N groups participated in the emission processes through their vibronic coupling. In the case of L = 4-ethylpyridine, the lifetime of emission was measured in the same temperature range, leading to the conclusion that the activation energy of the non-radiative processes (ΔEa) was estimated to be 20 kJ mol−1.
Introduction
Photoemitting metal complexes are attracting extensive attention due to their applicability to optical devices in the fields of sensing, memory and catalysis.1 The advantage of using metal complexes for such attempts lies in the fact that their emitting properties can be tuned by changing structural parameters systematically such as the derivatization of ligands and/or the change of valence states of metal ions.Some metal ions with nd10 electronic configurations are known to interact attractively, whether they are in the same or different molecules.2 The effects are denoted as aurophilic or argentophilic interactions for Au(I) or Ag(I) ions, respectively. In the case of Au(I) ions, for example, such interaction makes the interatomic distance shorter than the sum of van der Waals radii (0.36 nm). Quantum mechanically it is rationalized that (n + 1)p orbitals participate in the formation of a pair of bonding and anti-bonding orbitals as hybridized with nd orbitals.2 One remarkable feature is that the Au(I)–Au(I) pair is phosphorescent under the irradiation of UV light. A number of studies have been reported to develop emitting functional materials on the basis of multi-nuclear Au(I) complexes.3 Particular attention is paid to the possibility of tuning the emission maximum as a function of interatomic distance.4
Hofmann-type compounds are characterized by 2-D network sheets consisting of octahedral M(II) ions and square-planar [M(II)(CN)4] or linear [M(I)(CN)2] units.5 Each sheet is ligated by amine or pyridine derivatives coordinating with one or two octahedral M(II) ions. We have studied the spin cross-over of Hofmann-like compounds for a series of [MII(pyridine derivative)2][Au(CN)2]2. In the case of M(II) = Fe(II), for example, the magnetic transition from the high- to low-spin multiplet states was observed on lowering the temperature.6
We recently reported the emission properties of a Hofmann-type compound, [Cd (3-methylpyridine)2][Au(CN)2]2, in which Cd(II) was chosen as an octahedral M(II) ion because its filled d10 configuration eliminated the pathways of quenching via spin-multiplet states.7 As an extension of the study, the present work intended to establish the relationship between the polymer structures and the emission properties of bimetallic coordination polymers. For this purpose, a series of compounds with the elemental composition [CdIIL2][Au(CN)2]2(L = pyridine derivative) was synthesized and characterized by X-ray diffraction analyses. In all the prepared compounds, there existed a pair of Au(I)–Au(I) ions with the interatomic distance less than 0.36 nm. Emission spectra were recorded in the temperature range from 183 K to 363 K. The spectral profile of emission was found to be dependent remarkably on the structure of a ligated pyridine derivative.
Results and discussion
Preparation of a coordination polymer, [Cd(L)2]–[Au(CN)2]2 (L = pyridine derivative)
Chart 1 shows the molecular structures of pyridine derivatives used in the present work. The synthetic details are described in the Experimental section. The results of elemental analysis were consistent with the formula based on [CdIIL2][Au(CN)2]2 (ESI†). From the infra-red spectra of the KBr disk of the prepared compound, the stretching vibration of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups was observed at 2150–2170 cm−1 (ESI†). The wavenumber of the peak was higher than that observed for K[Au(CN)2] (2135 cm−1), assisting the view that the C
N groups was observed at 2150–2170 cm−1 (ESI†). The wavenumber of the peak was higher than that observed for K[Au(CN)2] (2135 cm−1), assisting the view that the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups of [Au(CN)2]− acted as a bidentate bridging ligand.8
N groups of [Au(CN)2]− acted as a bidentate bridging ligand.8
X-ray analyses
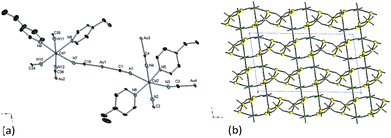
Single-crystal X-ray analyses were performed for the obtained crystals. The crystal structure of [Cd(3-methylpyridine)2][Au(CN)2]2 was reported previously.7 The data of the compound were collected additionally at 180 and 90 K. The space group was concluded to be (P21/c) in the whole temperature range. The compound belongs to a Hofmann-type compound with octahedral Cd(II) ions bridged by linear [Au(CN)2] moieties. The straight array of –CN–Cd(II)–NC–Au(I)–CN– runs in the diagonal direction of the yz-plane of the unit cell within one layer. The bilayer connections occur at the positions of two Au(I) atoms in the upper and lower layers. The interatomic distance of such Au(I) atoms is 0.31705(4) nm (298 K),7 0.31479(6) nm (180 K) and 0.31271 (3) nm (90 K), respectively.Fig. 1 shows the crystal structure of [Cd(4-ethylpyridine)2][Au(CN)2]2. There are two types of Cd(II) atoms: one Cd(II) coordinated with two 4-ethylpyridine molecules at trans-positions (denoted as trans-Cd(II)) and the other at cis-positions (cis-Cd(II)). The compound does not belong to a Hofmann-type compound. The reason for the formation of the cis-conformation around a Cd(II) ion might be sought in the absence of crystal field stabilization. In the case of Cd(II), the main force for the coordination interaction was electrostatic so that there might be little preference between trans- and cis-configurations of two coordinated 4-ethylpyridines. Accordingly, the steric interaction among ethyl groups destabilizes trans-Cd(II) ions, leading to the appearance of half of Cd(II) as a cis-configuration. trans-Cd(II) ions form a linear array by being connected with [Au(CN)2] moieties in the direction of the z-axis. cis-Cd(II) ions also form a linear array by being connected with [Au(CN)2] moieties in the direction of the y-axis. Alternatively connected trans- and cis-Cd(II) ions form a curved chain in the direction of the x-axis. These Cd[Au(CN)2]2 frameworks form a doubly interpenetrated 3-D network structure. There are four kinds of Au(I)–Au(I) pairs and three of them give the interatomic distance shorter than the sum of van der Waals radii or Au(1)–Au(2) = 0.30941(5) nm, Au(2)–Au(3) = 0.32467(5) nm and Au(3)–Au(4) = 0.31219(4) nm at 90 K. Crystallographic parameters are given in the ESI.† In order to confirm that the structure shown in Fig. 1 was unique for this compound, the powder diffraction pattern of the bulk sample (ca. 100 mg) was compared with the one calculated from the crystal structure (ESI†). Since they gave identical diffraction patterns in the measured region (0–50 degrees), the structure shown in Fig. 1 was concluded to be a unique case for this compound.
Fig. 2 shows the crystal structure of [Cd(3,5-lutidine)2][Au(CN)2]2. The compound crystallized in the space group Pca21. It belongs to the Hofmann-type compound. The bilayer structure is formed through the connection at two Au(I) atoms in the upper and lower layers as already seen for [Cd(3-methylpyridine)2][Au(CN)2]2. The distances of such Au(I) atoms are 0.31440(6) nm (298 K), 0.31419(6) nm (180 K) and 0.31345(5) nm (90 K), respectively.
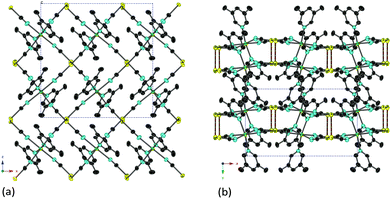 | ||
| Fig. 2 The crystal structure of [Cd(3,5-lutidine)2][Au(CN)2]2: (a) the projection along the y-axis; (b) the projection along the z-axis. | ||
Fig. 3 shows the crystal structure of [Cd(3-fluoropyridine)2][Au(CN)2]2. The compound crystallized in the space group P21/c. The compound belongs to the Hofmann-type compound. One of the two 3-fluoropyridine molecules coordinating a Cd(II) atom shows disorder so that it appears to be 3,5-difluoropyridine. The bilayer structure is formed through the connection at two Au(I) atoms in the upper and lower layers. The distance of such Au(I) atoms is 0.31874(11) nm (298 K).
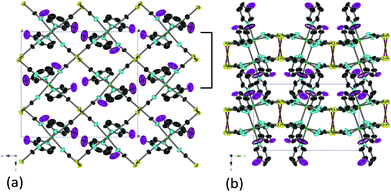 | ||
| Fig. 3 The crystal structure of [Cd(3-fluoropyridine)2][Au(CN)2]2: (a) the projection along the x-axis; (b) the projection along the z-axis. | ||
Steady-state emission properties
The emission and excitation spectra of the prepared compounds were recorded under air. The excitation wavelength was 370 nm when recording the emission spectra. A powder sample (∼50 mg) mounted in a quartz tube (φ 4 mm) was cooled down to 183 K and thereafter warmed gradually to 363 K at a rate of ca. 5 K min−1. The emission and excitation spectra of [Cd(3-methylpyridine)2][Au(CN)2]2 were reported previously.7 According to the results, the compound showed the emission in the wavelength region of 380–700 nm. The spectrum was characterized by the following two aspects: (i) several peaks were observed with nearly the same wavenumber interval (∼2000 cm−1) in both the emission and excitation spectra and (ii) the intensity of emission decreased remarkably on raising the temperature, accompanied by the change of the spectral shape. The excitation spectrum also changed its spectral shape on raising the temperature.Fig. 4 shows the emission spectra of [Cd(4-ethylpyridine)2][Au(CN)2]2, when the compound was irradiated at 370 nm in the temperature range from 183 K to 363 K. The spectra showed multiple peaks at 395, 430, 470 and 520 nm. In particular, the peak at 395 nm appeared only when the temperature was below 260 K. Since there existed three Au(I)–Au(I) pairs with different distances, it was possible that each pair gave different emission profiles with a vibrational structure. This made the spectral shape more complex.
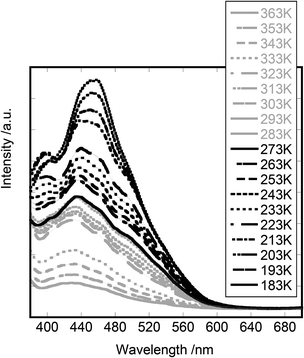 | ||
| Fig. 4 The emission spectra of [Cd(4-ethylpyridine)2][Au(CN)2]2: the excitation wavelength was 370 nm. The temperature was changed from 183 K to 363 K. | ||
Fig. 5 shows the emission spectra of [Cd(3,5-lutidine)2][Au(CN)2]2, when the compound was irradiated at 370 nm in the temperature range from 183 K to 363 K. At 183 K, the spectrum showed two main peaks at 420 and 465 nm. The emission intensity decreased over the whole wavelength region until temperature increased to 300 K. Above this temperature, however, the decrease of the peak at 420 nm was larger than that at 465 nm so that the spectral shape changed a little. The drastic change of the emission spectrum occurred at 323 K where the peak at 465 nm increased with a little increase of that at 420 nm. The former decreased rapidly above 323 K to 363 K, while the latter remained nearly at the same level. The possibility of structural change during the measurement of the emission spectrum was examined by comparing the powder X-ray patterns at room temperature before and after the measurements. As shown in the ESI,† there was no change of reflection patterns after the measurements. In order to see the reversibility of the above trend, the sample was cooled down to 135 K and warmed again to 373 K. No anomalous behaviour as stated above appeared and the emission decreased monotonously with the increase of temperature. It was noted that the initially mounted powder sample was cracked into finer particles during the first measurement. The irreversible change of crystal structures took place at higher temperatures (>323 K).
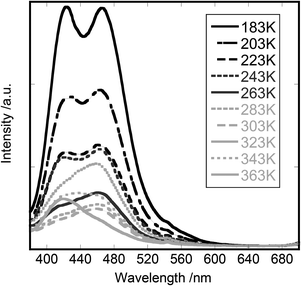 | ||
| Fig. 5 The emission spectra of [Cd(3,5-lutidine)2][Au(CN)2]2: the excitation wavelength was 370 nm. The temperature was changed from 183 K to 363 K. | ||
Fig. 6 shows the emission spectra of [Cd(3-fluoropyridine)2][Au(CN)2]2 in the temperature range from 183 K to 263 K. The peak at 445 nm decreased on raising the temperature from 183 K with the simultaneous blue shift until it was located at 435 nm at 273 K. In correspondence to this change, the broad shoulder was observed around 460 nm at higher temperatures.
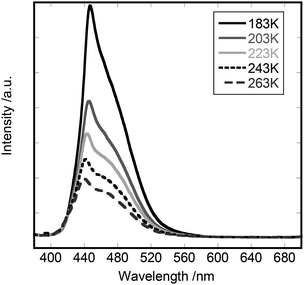 | ||
| Fig. 6 The emission spectra of [Cd(3-fluoropyridine)2][Au(CN)2]2: the excitation wavelength was 370 nm. The temperature was changed from 183 K to 263 K. | ||
Dynamic emission properties
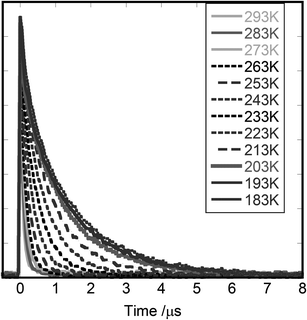
The lifetime of the emission was measured in the case of [Cd(4-ethylpyridine)2][Au(CN)2]2 over the temperature range of 183–363 K. Fig. 7 shows the decay curves of the emission monitored at 450 nm when the powder sample was irradiated by the laser pulse at 355 nm. The decay curve was analysed assuming that it consisted of two relaxation times as below:The values of fast (τ1) and slow (τ2) relaxation times are shown in the ESI† together with the amplitudes of F1 and F2. The decay rate increased on raising the temperature together with the decrease of emission intensity. It was assumed that the deactivation of the excited state occurred through two paths: one was spontaneous emission and the other non-radiative quenching. Based on this, the relaxation time was expressed by the following equation:
| 1/τi = 1/τi(se) + 1/τi(nr) (i = 1 or 2), |
A proposed mechanism of emission
The emission from the present coordination polymers was ascribed to the Au(I)–Au(I) pairs in the aurophilic interaction in crystals.8 The interatomic distance of a Au(I)–Au(I) pair was compared, and the distance varied at most by the amount of ∼0.015 nm. Such a small variation of the interatomic distance might not be a main factor causing the observed variation of emission spectra. The multiple peaks were assumed to represent the vibronic structures of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group.9 Thus the peak intensity reflected the population of each vibrational level.
N group.9 Thus the peak intensity reflected the population of each vibrational level.
Since the emission was phosphorescent, there occurred an intersystem crossing from an initially exited singlet state to an emitting triplet state. Supposing that the energy of an excited state is affected by the interatomic distance of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups coordinating Au(I) atoms, the potential energy diagram of the singlet and triplet states would be expressed as a function of the interatomic distance of a C
N groups coordinating Au(I) atoms, the potential energy diagram of the singlet and triplet states would be expressed as a function of the interatomic distance of a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group. Based on this scheme, the population distribution among the vibrational level of CN groups right after the intersystem crossing was dependent on the potential energy curves. The modification of a pyridine derivative might affect the position of the intersystem crossing through the induced structural change of the network of a coordination polymer. These situations lead to the variation of the spectral shape for the present series of compounds. This mechanism might open a possibility to tune the emission profile by modifying the structure of a ligand coordinating a Au(I) atom.
N group. Based on this scheme, the population distribution among the vibrational level of CN groups right after the intersystem crossing was dependent on the potential energy curves. The modification of a pyridine derivative might affect the position of the intersystem crossing through the induced structural change of the network of a coordination polymer. These situations lead to the variation of the spectral shape for the present series of compounds. This mechanism might open a possibility to tune the emission profile by modifying the structure of a ligand coordinating a Au(I) atom.
Experimental
Materials
The compounds were synthesized according to the following procedures.7,10 The appropriate amounts of monoethanolamine and citric acid were added to an aqueous solution (50 mL) containing CdCl2·2.5H2O (0.285 g, 1.23 mmol) and K[Au(CN)2] (0.720 g, 2.50 mmol) under stirring. When the pH was adjusted to ca. 10, the whole solution was transparent. Thereafter a part of the solution was placed as an inner vessel (∼10 mL) in an outer vessel (∼50 mL) containing a neat pyridine derivative. The inner solution was exposed to a vapour of a pyridine derivative for a few days, resulting in the colourless crystal at the bottom. The crystal was collected by filtration.Instruments
The structural characterization of the compounds was carried out by single crystal X-ray diffraction using a BRUKER APEXII SMART CCD area-detector diffractometer with monochromated Mo-Kα (λ = 0.071073 nm) under the temperature controlled N2 gas flow. The diffraction data were treated using APEX211 and SAINT,11 and absorption data were obtained using SADABS.12 The structures were solved by direct methods, expanded using Fourier techniques, and refined by full-matrix least-squares refinement. All non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were generated geometrically. All the structural solutions are performed using SHELXTL.13 Crystal data are listed in Tables S1 and S2 (ESI†).The emission spectra were recorded with a fluorometer FP-6500 (JASCO, Japan) equipped with a temperature-changing cell holder Unispeks, (UNISOKU, Japan). The life time of emission was measured with a TSP-1000M-PL-ES (UNISOKU, Japan). The instrument was equipped with a pulse YAG laser at 355 nm. The emission decay curve was obtained by averaging 130 pulsed signals. The curves were analysed under the assumption of multi-exponential decays.
Conclusions
The crystal structure and emission properties of a series of coordination polymers with the common composition of [CdII(pyridine derivative)2][Au(CN)2]2 were studied. All of the compounds contained an Au(I)–Au(I) pair with the aurophilic interaction. They were emissive under the irradiation of UV light (370 nm) and the emission spectra were characterized by multiple peaks with the spacing of 2000 cm−1. It was postulated that the emitting moieties contained C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups that contributed to the vibronic structures in emission. The results suggested the importance of the vibration modes in coordination ligands around in developing emitting devices based on coordination polymers.
N groups that contributed to the vibronic structures in emission. The results suggested the importance of the vibration modes in coordination ligands around in developing emitting devices based on coordination polymers.
Acknowledgements
The work was supported by MEXT (Ministry of Education, Culture, Sports, Science, and Technology, Japan)-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016. This work was also partly supported by JSPS KAKENHI Grant Number 15K05485. This work has been financially supported by the MEXT KAKENHI Grant-Aid-for Scientific Research (B) Number 26288039.Notes and references
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259 RSC; V. W.-W. Yam and K. M.-C. Wong, Chem. Commun., 2011, 47, 11579 RSC; Y. Shigeta, A. Kobayashi, T. Ohba, M. Yoshida, T. Matsumoto, H.-C. Chang and M. Kato, Eur. J. Chem., 2015, 21, 1 CrossRef CAS; H. Hosokawa and T. Mochida, Langmuir, 2015, 31, 13048 CrossRef PubMed.
- S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741 RSC; H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; E. R. T. Tiekink, Coord. Chem. Rev., 2014, 275, 130 CrossRef CAS; H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746 CrossRef PubMed; M. F. Espada, J. Campos, J. Lopez-Serrano, M. L. Poveda and E. Carmona, Angew. Chem., Int. Ed., 2014, 54, 1 Search PubMed; R. J. Roberts, D. Le and D. B. Leznoff, Chem. Commun., 2015, 51, 14299 RSC.
- E. E. Langdon-Jones and S. J. A. Pope, Chem. Commun., 2014, 50, 10343 RSC; C. Latouche, Y.-C. Lee, J.-H. Liao, E. Furet, J.-Y. Saillard, C. W. Liu and A. Boucekkine, Inorg. Chem., 2012, 51, 11851 CrossRef CAS PubMed; K. Fujisawa, S. Yamada, Y. Yanagsi, Y. Yoshida, A. Kiyohara and O. Tsutumi, Sci. Rep., 2015, 5, 7934 CrossRef PubMed.
- M. A. Rawasheh-Omary, J. M. Lopez-de-Luzuriagam, M. D. Rashdan, O. Elbjeriami, M. Monge, M. Rodrigurez-Castillo and A. Laguna, J. Am. Chem. Soc., 2009, 131, 3824 CrossRef PubMed; R. J. Robers, N. Bélanger-Desmarais, C. Reber and D. B. Lezoff, Chem. Commun., 2014, 50, 3148 RSC.
- K. Yoshida, D. Akahoshi, T. Kawasaki, T. Saito and T. Kitazawa, Polyhedron, 2013, 66, 252 CrossRef CAS; W. Liu, L. Wang, Y.-J. Su, Y.-C. Chen, J. Tucek, R. Zboril, Z.-P. Ni and M.-L. Tong, Inorg. Chem., 2015, 54, 8711 CrossRef PubMed.
- T. Kosone, C. Kanadani, T. Saito and T. Kitazawa, Polyhedron, 2009, 28, 1930–1934 CrossRef CAS.
- T. Kitazawa, K. Hiruma, H. Sato, K. Tamura and A. Yamagishi, Dalton Trans., 2013, 42, 16680 RSC.
- K. H. Kim, J. G. Kim, S. Nozawa, T. Sato, K. Y. Oang, T. W. Kim, H. Ki, J. Jo, S. Park, C. Song, T. Sato, K. Ogawa, T. Togashi, K. Tono, M. Yabashi, T. Ishikawa, J. Kim, R. Ryoo, J. Kim, H. Ihee and S. Adachi, Nature, 2015, 518, 385 CrossRef CAS PubMed.
- P. A. Tanner, X. Zhou, W.-T. Wong, C. Kratzer and H. Yersin, J. Phys. Chem. B, 2005, 109, 13083 CrossRef CAS PubMed; J. R. Thompson, M. J. Katz, V. E. Williams and D. B. Leznoff, Inorg. Chem., 2015, 54, 6462 CrossRef PubMed; V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589 CrossRef PubMed.
- S. Ueno, T. Kawasaki, J. Okabayashi and T. Kitazawa, Bull. Chem. Soc. Jpn., 2015, 88, 551 CrossRef CAS.
- Bruker, APEX2 and SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2007 Search PubMed.
- G. M. Sheldrick, SADABS, Empirical absorption correction program for area detector data, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2015, 71, 3 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Elemental analyses; IR analyses; crystal data; Cd[Au(CN)2]2 frameworks; the local structure of [Cd(3,5-lutidine)2{Au(CN)2}]2; PXRD of [Cd(4-ethylpyridine)2{Au(CN)2}2]n; temperature dependence of the decay rates; excitation spectra. CCDC 1448316–1448321 and 938995. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00537c |
| This journal is © The Royal Society of Chemistry 2016 |