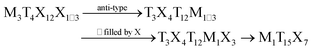Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
AlPd15B7: a new superconducting cage-compound with an anti-Yb3Rh4Sn13-type of structure†
Qiang
Zheng
a,
Walter
Schnelle
a,
Yurii
Prots
a,
Matej
Bobnar
a,
Ulrich
Burkhardt
a,
Andreas
Leithe-Jasper
a and
Roman
Gumeniuk
*ab
aMax-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Str. 40, 01187 Dresden, Germany. E-mail: roman.gumeniuk@physik.tu-freiberg.de
bInstitut für Experimentelle Physik, TU Bergakademie Freiberg, Leipziger Str. 23, 09596 Freiberg, Germany
First published on 2nd February 2016
Abstract
A new intermetallic compound AlPd15B7 was synthesized by arc-melting the stoichiometric mixture of the elements. Single crystal X-ray diffraction data of ternary metal-rich boride reveal a new type of structure with the space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d and the lattice parameter a = 16.4466(3) Å. It adopts a filled anti-Yb3Rh4Sn13-type structure, where the positions corresponding to 3Yb, 4Rh and 13Sn atoms are occupied by 3Pd, 4B, and 1Al + 12 Pd, respectively and 3B additionally at interstitial sites. Magnetic susceptibility, electrical resistivity, and specific heat measurements reveal bulk superconductivity with a critical temperature Tc ≈ 2.9 K. Electronic structure calculations show that Pd 4d and B 2p states dominate the density of states (DOS) at the Fermi level EF.
d and the lattice parameter a = 16.4466(3) Å. It adopts a filled anti-Yb3Rh4Sn13-type structure, where the positions corresponding to 3Yb, 4Rh and 13Sn atoms are occupied by 3Pd, 4B, and 1Al + 12 Pd, respectively and 3B additionally at interstitial sites. Magnetic susceptibility, electrical resistivity, and specific heat measurements reveal bulk superconductivity with a critical temperature Tc ≈ 2.9 K. Electronic structure calculations show that Pd 4d and B 2p states dominate the density of states (DOS) at the Fermi level EF.
Introduction
In the early 1980s, Remeika and co-workers discovered a family of M3T4X13 cage-compounds (where M = rare-earth, alkaline-earth, or actinide metal, T = transition metal, and X = In or group-IV elements) crystallizing with a primitive cubic Yb3Rh4Sn13-type structure (space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n).1–4 They became an object of numerous studies, mostly owing to the interesting interplay between magnetic and superconducting properties.1,5–9 The crystal structures of both Remeika M3T4X13 phases and filled-skutterudites10,11 are derivatives of the simple perovskite-type structure, and show similar arrays of corner-sharing trigonal prisms/antiprisms (octahedra) enclosing icosahedral voids. For more details about their structural relationships, we refer to ref. 12 (and references therein). In recent studies,12–14 several new cubic, tetragonal, rhombohedral and monoclinic distorted variants of the Remeika prototype with a Pt–Ge framework were reported. In these structures, distortions occur exclusively in the Ge-framework, while M and Pt atoms remain in their original positions. In the M3T4X13 structures, twelve X atoms form icosahedra filled by one remaining X atom, T atoms reside in trigonal prisms formed by two neighboring [X12] icosahedra, and M atoms are encapsulated in cubooctahedra built by X atoms (see Fig. S3 in the ESI†). Owing to the variety of M, T, and X elements forming such compounds without major changes in the crystal structures, substitution by elements not only limited to the above mentioned groups should be possible.
n).1–4 They became an object of numerous studies, mostly owing to the interesting interplay between magnetic and superconducting properties.1,5–9 The crystal structures of both Remeika M3T4X13 phases and filled-skutterudites10,11 are derivatives of the simple perovskite-type structure, and show similar arrays of corner-sharing trigonal prisms/antiprisms (octahedra) enclosing icosahedral voids. For more details about their structural relationships, we refer to ref. 12 (and references therein). In recent studies,12–14 several new cubic, tetragonal, rhombohedral and monoclinic distorted variants of the Remeika prototype with a Pt–Ge framework were reported. In these structures, distortions occur exclusively in the Ge-framework, while M and Pt atoms remain in their original positions. In the M3T4X13 structures, twelve X atoms form icosahedra filled by one remaining X atom, T atoms reside in trigonal prisms formed by two neighboring [X12] icosahedra, and M atoms are encapsulated in cubooctahedra built by X atoms (see Fig. S3 in the ESI†). Owing to the variety of M, T, and X elements forming such compounds without major changes in the crystal structures, substitution by elements not only limited to the above mentioned groups should be possible.
In this study, we report the boride AlPd15B7 with a new type of structure, which is closely related to the Yb3Rh4Sn13 prototype. Physical property measurements reveal superconducting behavior of this new boride with Tc ≈ 2.9 K. The thermodynamic properties are compatible with an s-wave energy gap conventional superconductivity and moderate electron–phonon coupling.
Experimental section
Sample preparation
The samples with a nominal composition of AlPd15B7 were synthesized by arc-melting of aluminum (Chempur, 99.9999 mass%) and palladium (Chempur, 99.9 mass%) foils and crystalline boron powder (Alfa Aesar, 99.999 mass%). The obtained button was remelted several times to obtain a homogeneous sample. The weight loss during arc-melting was less than 0.1 mass%. All above operations were performed inside an argon-filled glove box (p(O2/H2O) ≤ 1 ppm). A nearly single phase sample was obtained. The resulting sample is stable in air for long time.Powder and single-crystal X-ray diffraction
Due to the ductility, stress annealing of ground powders was performed at 840 °C for 1.5 h to obtain powder diffraction patterns with sharper and better resolved diffraction peaks. Powder X-ray diffraction (XRD) data were collected on a HUBER G670 imaging plate Guinier camera (Cu Kα1 radiation, λ = 1.540598 Å). Phase analysis and indexing were performed within the WinXPow program package.15 Lattice parameters were refined by least-squares fitting with LaB6 internal standard correction within the program package WinCSD.16Single crystals were selected from the stress-annealed crushed sample. Single crystal XRD data were collected on a Rigaku AFC7 diffraction system equipped with a Saturn 724+ CCD detector (Mo Kα radiation, λ = 0.71073 Å). Absorption correction was made using a multi-scan procedure. The crystal structure was solved by a direct phase determination method and refined by a full-matrix least-squares procedure within the program package WinCSD.16 Details of the single crystal XRD data collection are listed in Table 1.
| Composition | AlPd15B7 |
|---|---|
| Space group |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d d |
| Lattice parameters | |
| a (Å) | 16.4466(3) |
| V (Å3) | 4448.7(3) |
| Formula unit per cell, Z | 16 |
| Calculated density (g cm−3) | 10.14 |
| Diffraction system | Rigaku AFC7 |
| Radiation; λ (Å) | Mo Kα; 0.71073 Å |
| 2θmax (°) | 67.07 |
| Absorption coefficient (mm−1) | 23.07 |
| N(hkl)measured | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108 |
| N(hkl)unique | 730 |
| N(hkl)observed (Fhkl > 4σ(F)) | 718 |
| R int/Rσ | 0.053/0.037 |
| Refined parameters | 32 |
| R F/wRF2 | 0.058/0.064 |
| Extinction coefficient | 0.00026(2) |
| Residual electron density (e Å−3) | +1.1; −0.8 |
Metallography
A small piece of the as-cast AlPd15B7 sample was embedded in a conductive resin and then subjected to a multistep grinding and polishing process to achieve a high-quality surface. The microstructure observations were performed on an optical microscope (Axioplan 2, Zeiss) as well as on a scanning electron microscope (JSM-7800F, JEOL). The chemical composition of the observed phase was analysed by means of energy dispersive X-ray spectroscopy (EDXS, Quantax 400 EDXS system, Silicon Drift Detector, Bruker) and wavelength dispersive X-ray spectroscopy (WDXS, SX 100, Cameca) using Al Kα, Pd Lα, and B Kα signals with elemental Al and Pd3B as standards. The Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd atomic ratio of the studied phase from EDXS and its composition from WDXS were measured to be 0.9(1)
Pd atomic ratio of the studied phase from EDXS and its composition from WDXS were measured to be 0.9(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.0(1) and Al4.6(2)Pd66.5(2)B28.9(1), respectively, in good accordance with the theoretical values (1
15.0(1) and Al4.6(2)Pd66.5(2)B28.9(1), respectively, in good accordance with the theoretical values (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and Al4.35Pd65.22B30.43, respectively).
15 and Al4.35Pd65.22B30.43, respectively).
Physical properties
The magnetization data of a polycrystalline AlPd15B7 sample were collected in external fields μ0H ranging from 2 mT to 7 T and temperatures between 1.8 K and 400 K on a SQUID magnetometer (MPMS-XL7, Quantum Design). The electrical resistivity was measured using a four-point ac method between 1.8 K and 320 K (PPMS, Quantum Design) on a small bar (1 mm × 1 mm × 6.5 mm). The heat capacity was measured by means of a relaxation-type calorimeter on a PPMS in external fields μ0H up to 0.5 T between 0.35 and 320 K.Electronic structure calculations
The scalar-relativistic band-structure of AlPd15B7 was calculated within the local-density approximation (LDA) of density functional theory (DFT) using the full-potential local-orbital FPLO code (version 9.01-35).17 The calculation was performed by employing the exchange-correlation potential of Perdew and Wang18 and using the experimental structural data from Table 2. The first Brillouin zone was sampled by a mesh of 12 × 12 × 12 (1728) k points.| Atom | Site | x | y | z | B iso/Beqa |
|---|---|---|---|---|---|
a
B
eq = 1/3[a*2a2B11 + b*2b2B22 + c*2c2B33 + 2aba*b*(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ)B12 + 2aca*c*(cos γ)B12 + 2aca*c*(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β)B13 + 2bcb*c*(cos β)B13 + 2bcb*c*(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α)B23]. α)B23].
|
|||||
| Al | 16a | 0 | 0 | 0 | 1.08(8) |
| Pd1 | 48g | 1/8 | 0.24484(6) | 0.00516(6) | 1.23(2) |
| Pd2 | 96h | 0.00090(6) | 0.14693(6) | 0.07718(6) | 0.99(2) |
| Pd3 | 96h | 0.10758(6) | 0.24514(6) | 0.33770(6) | 1.13(2) |
| B1 | 16b | 1/8 | 1/8 | 1/8 | 1.4(5) |
| B2 | 48f | 0.263(2) | 0 | 1/4 | 1.5(3) |
| B3 | 48g | 1/8 | 0.619(1) | 0.631(1) | 1.4(3) |
Results and discussion
Crystal structure determination
As shown in Fig. 1 and Fig. S1 (in the ESI†), the XRD patterns of the ground powders of an as-cast AlPd15B7 sample with and without stress-annealing reveal no difference between them besides peaks broadening in the latter one. As revealed by Fig. S2 in the ESI,† the content of impurities in the as-cast sample is less than 1 vol%. The strong reflections could be indexed in a simple cubic unit cell with lattice parameter a = 8.2233(2) Å. However, as one can see as an example in the inset of Fig. 1, there are still many reflections that could not be indexed in this unit cell, revealing the appearance of a superstructure. Taking these reflections into account, a body centered cubic unit cell with lattice parameter a = 16.4466(3) Å is obtained. Subsequently, single crystals were selected for XRD experiments.Details on single crystal XRD data collection for AlPd15B7 are listed in Table 1. Analysis of the reflection intensities and the extinction conditions confirm the appearance of the superstructure, indicating centrosymmetry and only one possible space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d. Positions of the heavy Pd and Al atoms and the light B atoms were acquired by a direct phase determination procedure and from difference Fourier maps, respectively. Anisotropic displacement parameters for all the Al and Pd atoms were refined. Final atomic coordinates and anisotropic atomic displacement parameters are listed in Tables 2 and 3, respectively. Moreover, diffraction data were also collected for single crystals selected from powders of the as-cast sample without stress annealing, and reveal the same structure solution result and only larger residual values due to broadening of the reflections. The acquired composition is in good agreement with the compositions from the EDXS and WDXS analysis.
d. Positions of the heavy Pd and Al atoms and the light B atoms were acquired by a direct phase determination procedure and from difference Fourier maps, respectively. Anisotropic displacement parameters for all the Al and Pd atoms were refined. Final atomic coordinates and anisotropic atomic displacement parameters are listed in Tables 2 and 3, respectively. Moreover, diffraction data were also collected for single crystals selected from powders of the as-cast sample without stress annealing, and reveal the same structure solution result and only larger residual values due to broadening of the reflections. The acquired composition is in good agreement with the compositions from the EDXS and WDXS analysis.
| Atom | B 11 | B 22 | B 33 | B 12 | B 13 | B 23 |
|---|---|---|---|---|---|---|
| Al | 1.1(1) | B 11 | B 11 | −0.06(15) | B 12 | B 12 |
| Pd1 | 1.09(5) | 1.31(4) | B 22 | −0.11(3) | B 12 | 0.12(4) |
| Pd2 | 0.93(4) | 1.09(4) | 0.96(4) | 0.01(2) | −0.02(2) | 0 |
| Pd3 | 1.26(4) | 1.08(4) | 1.06(4) | −0.09(3) | −0.02(3) | −0.14(3) |
Crystal structure
AlPd15B7 crystallizes with a new type of structure, which can be regarded as a filled anti-Yb3Rh4Sn13 structure. The crystal structures of AlPd15B7 and Yb3Rh4Sn13 are shown in Fig. 2a and Fig. S3 in the ESI,† respectively. The group–subgroup relationship between them is shown in Fig. 2b. The crystallographic positions for 3Yb, 4Rh and 13Sn atoms in the Yb3Rh4Sn13 structure1 are now occupied by 3Pd, 4B, and 1Al + 12Pd atoms in the AlPd15B7 structure, respectively. The octahedra formed by Yb and Sn atoms are empty in the Yb3Rh4Sn13-type structure, whereas the corresponding octahedra solely formed by Pd atoms are filled by additional B atoms in the AlPd15B7 structure. The transformation between these two types of structures can be described as:where M, T, and X are Yb, Rh, and Sn in Yb3Rh4Sn13, and Al, Pd and B in AlPd15B7. Different from Yb3Rh4Sn13, the lattice parameters of AlPd15B7 are doubled and the space group changes from Pm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n to Ia
n to Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d, due to the shift of the Pd atoms from some special positions. As one can see in Fig. 6 in ref. 13, the relationship between many derivatives of the primitive cubic Yb3Rh4Sn13-type of structure (e.g. La3Rh4Sn13 (I4132), Ca3Pt4+xGe13−y (I213),12 HT-Y3Pt4Ge13 (R3c),13 Yb3Pt4Ge13 (P42cm),12 and LT-Y3Pt4Ge13 (Cc)14) can be understood within a group–subgroup scheme. AlPd15B7 crystallizes with the space group Ia
d, due to the shift of the Pd atoms from some special positions. As one can see in Fig. 6 in ref. 13, the relationship between many derivatives of the primitive cubic Yb3Rh4Sn13-type of structure (e.g. La3Rh4Sn13 (I4132), Ca3Pt4+xGe13−y (I213),12 HT-Y3Pt4Ge13 (R3c),13 Yb3Pt4Ge13 (P42cm),12 and LT-Y3Pt4Ge13 (Cc)14) can be understood within a group–subgroup scheme. AlPd15B7 crystallizes with the space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d, which links the space groups Pm
d, which links the space groups Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n and I4132 via the indices of k4 (klassengleiche) and t2 (translationengleiche) symmetry reductions, respectively.
n and I4132 via the indices of k4 (klassengleiche) and t2 (translationengleiche) symmetry reductions, respectively.
 | ||
| Fig. 2 (a) Crystal structure of AlPd15B7. For a better visualization, only a part of the structure in one unit cell is shown. [Al@Pd12] icosahedra – light gray; distorted [B@Pd6] trigonal prisms – dark gray; [B@Pd6] octahedra – blue. (b) group–subgroup relationship between the structures of Yb3Rh4Sn13 and AlPd15B7 (see text). For better visualization the coordinates for AlPd15B7 are not standardized, like those given in Table 2. | ||
The AlPd15B7 structure is shown in Fig. 2a, and interatomic distances are listed in Table S1 in the ESI.† In such a structural arrangement, similar to Al residing in [Ni12] icosahedra in the AlNi9B8 structure,19 each Al atom resides in a distorted [Pd12] icosahedron, consisting of 6Pd2 and 6Pd3 atoms, with the Al–Pd and Pd–Pd distances ranging from 2.730 Å to 2.752 Å, and from 2.714 Å to 2.955 Å, respectively. The empty spaces between the icosahedra are occupied by the Pd1, B1, B2 and B3 atoms. Each [Al@Pd12] icosahedron interconnects with 14 neighboring [Al@Pd12] icosahedra via 8 distorted corner-sharing trigonal prisms and 6 distorted octahedra. The two sorts of corner-sharing [Pd6] trigonal prisms, formed by 6 Pd2 atoms or 2Pd2 and 4Pd3 atoms, are centered by the B1 or B3 atoms, respectively. In a [B1Pd6] trigonal prism, the Pd2–Pd2 distances are 2.850 Å and 2.942 Å, and the Pd–B distance is 2.217 Å; in a [B3Pd6] trigonal prism, the Pd–Pd distances are in the range of 2.885 Å to 2.955 Å, the Pd2–B3 distance is 2.139 Å, and the Pd3–B3 distances are 2.157 Å and 2.28 Å. Each [Pd6] octahedron, formed by 2Pd1, 2Pd2, and 2Pd3 atoms, is centered by a B2 atom, with Pd–Pd distances and Pd–B distances ranging from 2.714 Å to 3.5423 Å and from 2.08 Å to 2.252 Å, respectively. These kinds of metal–atom coordination of boron are commonly observed in borides.20,21 In Pd3B (Fe3C type of structure),22 B resides in trigonal prisms with Pd–B distances of 2.166 Å to 2.180 Å; in Pd2B (anti-CaCl2 type of structure),23 B centers Pd octahedra with Pd–B distances of 2.104 Å to 2.122 Å. In comparison with the sum of atomic radii of pure elements (rB = 0.83 Å, rAl = 1.43 Å, and rPd = 1.38 Å (ref. 24)), the Al–Pd distances are shorter than the sum of atomic radii of Al and Pd, the Pd–Pd distances obviously deviate from the two-fold atomic radius of Pd, and the shortest and longest Pd–B distances are 6% shorter and ∼3% longer than the sum of atomic radii of Pd and B, respectively. Coordination polyhedra of all the atoms in AlPd15B7 are shown in Fig. S4 in the ESI.†
Physical properties
The magnetic susceptibility of AlPd15B7 in an external field of μ0H = 7 T is shown in the inset of Fig. 3, revealing that it is a diamagnet with an extrapolated value of χ0 = −2.0 × 10−4 emu mol−1 at T = 0. The slight upturn towards low temperature is probably due to minor paramagnetic impurities or point defects.The temperature dependence of the zero-field-cooled (zfc) and field-cooled (fc) magnetic susceptibility of AlPd15B7 in an external field of μ0H = 2 mT is given in Fig. 3, indicating a superconducting transition. The transition temperature was determined via a tangent to the steepest slope of χzfc(T), resulting in Tcmag = 2.78 K. Considering the demagnetization correction, the diamagnetic response in zfc is close to complete. The fc signal (Meißner effect) is much weaker, which is due to strong flux line pinning in the type-II superconductor (see below).
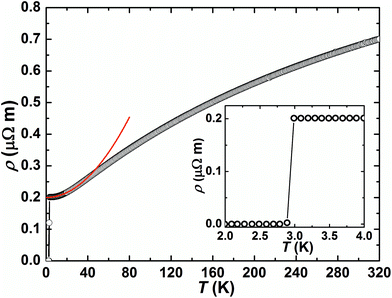
As shown in Fig. 4, the electrical resistivity of AlPd15B7 increases with increasing temperature in the range of 3.0–320 K, revealing a metallic behavior of this compound. For 4 K < T < 40 K, it can be fitted to ρ0 + AFLT2 with a residual resistivity ρ0 = 0.20(1) μΩ m from impurity scattering, and the cross section of quasiparticle–quasiparticle scattering AFL = 3.96(3) × 10−5 μΩ m K−2. The room-temperature resistivity ρ(300 K) is 0.68 μΩ m, and the residual resistivity ratio RRR = 3.4 indicates moderate quality of the investigated polycrystalline sample. As revealed by the inset of Fig. 4, a sharp superconducting transition can be observed, and the resistivity drops to zero at T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) resc = 2.89 K.
resc = 2.89 K.
The specific heat cp/T vs. T for AlPd15B7 in the temperature range 0.35–4.0 K and in various magnetic fields is shown in Fig. 5a. For zero-field, a sizable sharp step-like anomaly confirms the bulk nature of the superconductivity. The normal-state specific heat (Fig. 5b) can be described by cp,n(T) = γtotT + βT3 + δT5 in the temperature range 0.5–5 K, where γtot is the Sommerfeld coefficient of the electronic heat capacity, and βT3 + δT5 are the first terms of the harmonic lattice approximation for the phonon contribution. The resulting parameters are γtot = 28.3(1) mJ mol−1 K−2 and β = 1.38(2) mJ mol−1 K−4, which corresponds to an initial Debye temperature ΘD(0) = 319 K, and δ = 2.86(6) × 10−5 mol−1 K−6.
The electronic contribution cel(T, H) below Tc can be analyzed by subtracting the lattice terms βT3 + δT5. Fig. 5c shows Δcp = cel – γtotT which is well described by the BCS theory (for weak electron–phonon coupling) in the whole temperature range. The resulting parameters are the jump Δcp/Tc = 37.5 mJ mol−1 K−2, the transition midpoint Tcalc = 2.796 K, and the width of the transition of 0.026 Tc. The ratio Δcp/(γtotTc) of 1.32 is somewhat lower than the value 1.43 from the BCS theory. At the lowest temperatures (in the range 0.35–0.70 K) cel(T) is well fitted by  (as shown in Fig S5a in the ESI†). Such exponential behavior is expected for an s-wave superconductor without nodes of the gap, as in the BCS theory. The resulting parameters for zero field are γ0 = 2.17 mJ mol−1 K−2 and the energy gap ratio Δ/kBTc = 1.08. The non-zero γ0 is probably due to the presence of a non-superconducting metallic impurity phase. We may thus correct the normal state electronic specific heat coefficient by γn = γtot − γ0. The corrected value for the jump Δcp/(γnTc) = 1.44 reproduces even better the weak-coupling BCS value 1.43. However, the energy gap ratio Δ/kBTc observed at the lowest temperatures is much smaller than expected from BCS theory (Δ/kBTc = 1.76). It is therefore possible that AlPd15B7 needs to be described within a two-gap model. The resolution of the present specific heat data, due to the small available mass for the study, is insufficient for a more sophisticated analysis.
(as shown in Fig S5a in the ESI†). Such exponential behavior is expected for an s-wave superconductor without nodes of the gap, as in the BCS theory. The resulting parameters for zero field are γ0 = 2.17 mJ mol−1 K−2 and the energy gap ratio Δ/kBTc = 1.08. The non-zero γ0 is probably due to the presence of a non-superconducting metallic impurity phase. We may thus correct the normal state electronic specific heat coefficient by γn = γtot − γ0. The corrected value for the jump Δcp/(γnTc) = 1.44 reproduces even better the weak-coupling BCS value 1.43. However, the energy gap ratio Δ/kBTc observed at the lowest temperatures is much smaller than expected from BCS theory (Δ/kBTc = 1.76). It is therefore possible that AlPd15B7 needs to be described within a two-gap model. The resolution of the present specific heat data, due to the small available mass for the study, is insufficient for a more sophisticated analysis.
The DFT calculations reveal a DOS at the Fermi level N(EF) = 7.9 states eV−1 f.u.−1 (see below), converting to a “bare” Sommerfeld coefficient of the electronic specific heat γbare = 18.6 mJ mol−1 K−2. The electron–phonon coupling parameter λ in the relation γN = (1 + λ) γbare is calculated to be 0.40. λ can also be estimated from the McMillan formula ln(1.45Tc/ΘD) = −1.04 (1 + λ)/[λ − μ*(1 + 0.62λ)]. Assuming the common value μ* = 0.15, λ results as 0.58, which is somewhat larger than the above value.
The fitted Sommerfeld parameters γ(H) − γ0, extrapolated to T = 0, were plotted against the fields (Fig. S5b in the ESI†), showing a linear relationship with the magnetic fields. This also indicates a typical s-wave gap for AlPd15B7. The upper critical field values were estimated from the midpoints of superconducting transition in cp(T, μ0H), as shown in Fig. 5d. It is obvious that Tc(μ0H) varies almost linearly with μ0H. This most simple extrapolation results in μ0Hc2(0) ≈ 500(100) mT.
Electronic structure
The total and partial (atomic and orbital resolved) density of states (DOS) for AlPd15B7 are shown in Fig. 6(a–c). Similar to the electronic structures of the M3Pt4Ge13 compounds,12,13 the Fermi level EF for AlPd15B7 is located close to a dip in the DOS. Thus, the system is a metal with 7.9 states eV−1 f.u.−1 at EF. This DOS is mostly due to the contributions of the Pd 4d (∼71%) and the B 2p (∼25%) states. The broad valence band (∼7 eV) in the electronic structure of AlPd15B7 is dominated by Pd 4d states (Fig. 6b) with sizeable B 2p contributions (Fig. 6c) at lower energies (between −7 eV and −3.5 eV). Furthermore, the lowest lying separate band (between −11 eV and −7.5 eV) is mainly formed by B 2s and Pd 4d states. All this is related to the covalent bonding in the Pd–B framework. Al contributions to the valence band are negligibly small. Interestingly, a narrow separate band (∼0.3 eV) centered at −7.1 eV is mostly due to 4d states of Pd2 and Pd3 as well as Al 3s. Obviously, it reflects covalent interaction of Al and Pd atoms within the [Al@Pd12] icosahedra (Fig. 2). The electronic band structure of AlPd15B7 is reminiscent of the electronic structures of such Pd-rich borides as Pd3B and SrPd4B.25 They also possess a broad valence band dominated by Pd 4d and B 2p states followed by a separate low energy band due to the mixing of B 2s and Pd 4d states. The differences are that for AlPd15B7 a larger DOS at EF as well as an additional narrow band at −7.1 eV are observed. | ||
| Fig. 6 (a) Total and atom resolved electronic density of states (DOS) for AlPd15B7. Orbital resolved DOS for (b) palladium and (c) boron atoms. | ||
Conclusions
We gratefully acknowledge Prof. Yu. Grin's interest and steady support. A new ternary boride AlPd15B7, with a filled anti-Yb3Rh4Sn13-type structure, has been synthesized by arc-melting mixtures of elements, and its crystal structure was determined from the single-crystal X-ray diffraction data. In this structure, the Al atoms reside in the [Pd12] icosahedra, and each icosahedron interconnects with 14 neighboring [Pd12] icosahedra, forming 8 corner-sharing trigonal prisms and 6 octahedra, which are all filled by the B atoms. In comparison, the corresponding octahedra are empty in the Yb3Rh4Sn13-type structure.Magnetic susceptibility, electrical resistivity, and specific heat measurements show a superconducting transition of AlPd15B7 at a Tc of about 2.9 K. The specific heat measurements reveal AlPd15B7 to be a conventional s-wave superconductor with weak electron–phonon coupling according to the BCS theory. Electronic structure calculations by DFT reveal that the DOS at EF is dominated by d states.
AlPd15B7 is the first boride with a crystal structure comprising the framework of the Remeika Yb3Rh4Sn13 prototype but being decorated by completely different types of elements. The occurrence of this new type of structure suggests that the constituting elements in the Remeika structure framework could be extended to a variety of elements in future investigations.
Acknowledgements
We thank Mr S. Hückmann and Dr H. Borrmann for performing powder X-ray diffraction measurements, Ms. P. Scheppan, Ms. M. Eckert and Ms. S. Kostmann for the metallographic analysis, and Mr R. Koban for physical property measurements.References
- J. L. Hodeau, J. Chenavas, M. Marezio and J. P. Remeika, Solid State Commun., 1980, 36, 839 CrossRef CAS.
- P. Villars, Pearson's Handbook, Crystallographic Data for Intermetallic Phases, Materials Park, OH, 1997 Search PubMed.
- J. M. Vandenberg, Mater. Res. Bull., 1980, 15, 835 CrossRef CAS.
- S. Miraglia, J. L. Hodeau, M. Marezio, C. Laviron, M. Ghedira and G. P. Espinosa, J. Solid State Chem., 1986, 63, 358 CrossRef CAS.
- G. K. Shenoy, B. D. Dunlap and F. Y. Fradin, Ternary Superconductors, Elsevier, North-Holland, Amsterdam, 1981 Search PubMed.
- J. P. Remeika, G. P. Espinosa, A. S. Cooper, H. Barz, J. M. Rowell, D. B. McWhan, J. M. Vandenberg, D. E. Moncton, Z. Fisk, L. D. Woolf, H. C. Hamaker, M. B. Maple, G. Shirane and W. Thomlinson, Solid State Commun., 1980, 34, 923 CrossRef CAS.
- Y. Mudryk, A. Grytsiv, P. Rogl, C. Dusek, A. Galatanu, E. Idl, H. Michor, E. Bauer, C. Godart, D. Kaczorowski, L. Romaka and O. Bodak, J. Phys.: Condens. Matter, 2001, 13, 7391 CrossRef CAS.
- N. Kase, H. Hayamizu and J. Akimitsu, Phys. Rev. B: Condens. Matter, 2011, 83, 184509 CrossRef.
- B. K. Rai, I. W. H. Oswald, J. K. Wang, G. T. McCandless, J. Y. Chan and E. Morosan, Chem. Mater., 2015, 27, 2488 CrossRef CAS.
- W. Jeitschko and D. Braun, Acta Crystallogr., 1977, 33, 3401 CrossRef.
- R. Gumeniuk, H. Borrmann, A. Ormeci, H. Rosner, W. Schnelle, M. Nicklas, Yu. Grin and A. Leithe-Jasper, Z. Kristallogr., 2010, 225, 531 CrossRef CAS.
- R. Gumeniuk, L. Akselrud, K. O. Kvashnina, W. Schnelle, A. A. Tsirlin, C. Curfs, H. Rosner, M. Schöneich, U. Burkhardt, U. Schwarz, Yu. Grin and A. Leithe-Jasper, Dalton Trans., 2012, 41, 6299 RSC.
- R. Gumeniuk, M. Schöneich, K. O. Kvashnina, L. Akselrud, A. A. Tsirlin, M. Nicklas, W. Schnelle, O. Janson, Q. Zheng, C. Curfs, U. Burkhardt, U. Schwarz and A. Leithe-Jasper, Dalton Trans., 2015, 44, 5638 RSC.
- R. Gumeniuk, M. Nicklas, L. Akselrud, W. Schnelle, U. Schwarz, A. A. Tsirlin, A. Leithe-Jasper and Yu. Grin, Phys. Rev. B, 2013, 87, 224502 CrossRef.
- WinXPow, STOE and Cie GmbH, Darmstadt, 2003 Search PubMed.
- L. Akselrud and Yu. Grin, J. Appl. Crystallogr., 2014, 47, 803 CrossRef CAS.
- K. Koepernik and H. Eschrig, Phys. Rev. B, 1999, 59, 1743 CrossRef CAS.
- J. P. Perdew and Y. Wang, Phys. Rev. B, 1992, 45, 13244 CrossRef.
- Q. Zheng, F. R. Wagner, A. Ormeci, Yu. Prots, U. Burkhardt, M. Schmidt, W. Schnelle, Yu. Grin and A. Leithe-Jasper, Chem. – Eur. J., 2015, 21, 16532 CrossRef CAS PubMed.
- Yu. B. Kuz'ma, Crystallochemistry of Borides, Lviv University Publishers, Lviv, 1983 Search PubMed.
- P. Rogl, in Inorganic Reactions and Methods, ed. A. P. Hagen, Wiley, 1991 Search PubMed.
- E. Stenberg, Acta Chem. Scand., 1961, 15, 861 CrossRef CAS.
- L.-E. Tergenius and T. Lundström, J. Solid State Chem., 1980, 31, 361 CrossRef CAS.
- J. Emsley, The Elements, Clarendon Press, Oxford, 1998 Search PubMed.
- R. Gumeniuk, M. Schmitt, W. Schnelle, U. Burkhardt, H. Rosner and A. Leithe-Jasper, Z. Anorg. Allg. Chem., 2010, 636, 954 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1440657. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt04751j |
| This journal is © The Royal Society of Chemistry 2016 |