Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Half-sandwich nickel complexes with ring-expanded NHC ligands – synthesis, structure and catalytic activity in Kumada–Tamao–Corriu coupling†
Ł.
Banach
,
P. A.
Guńka
 and
W.
Buchowicz
*
and
W.
Buchowicz
*
Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: wbuch@ch.pw.edu.pl
First published on 28th January 2016
Abstract
The general synthesis of [Ni(Cp)(X)(NHC)] complexes from a nickel halide, CpLi, and a carbene solution is reported. This procedure yields unprecedented complexes with ring-expanded NHC ligands (RE-NHC) of six- (1a, 1b), seven- (1c), and eight-membered (1d) heterocycles. The NMR spectra of 1a–1d are consistent with the hindered rotation of Ni–Ccarbene and N–CMes bonds, while X-ray analyses of 1b, 1c, and 1d reveal a pronounced trans influence of the RE-NHC ligands. Complexes 1a–1e are efficient pre-catalysts in Kumada–Tamao–Corriu coupling with the maximum efficiency observed for complexes bearing the six-membered NHC.
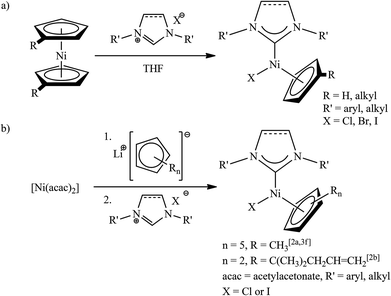
Half-sandwich nickel complexes of the general formula [Ni(η5-C5H4R)(X)(NHC)] (R = H, alkyl, alkenyl; X = Cl, Br, I; NHC = N-heterocyclic carbene) are known only for five-membered NHCs. These complexes are usually synthesized by reactions of nickelocene with imidazolium halides (Scheme 1a).1 However, for compounds with bulky substituents on the cyclopentadienyl (Cp) ligand, another protocol has to be utilized (Scheme 1b).2
Owing to their catalytic activity in many organic transformations, these air- and moisture-stable complexes are currently the object of intensive research.2–5 Efforts for tuning the properties of [Ni(Cp)(X)(NHC)] are focused on appropriate ligand selection within the scope of synthetic methodologies presented in Scheme 1, i.e. for five-membered NHCs only. Since the halide1a,3b–d,4 or Cp ligands5 can be readily displaced from the title complexes, NHC is the ligand of choice for modifications aimed at enhancing their catalytic activity. Recently, it has been shown that increasing the steric demand of NHCs by means of bulky substituents on the nitrogen atoms improved the efficiency of the arylamination of aromatic halides catalysed by [Ni(Cp)(X)(NHC)].3i Another way to enhance spatial requirements as well as the electron-donating strength of NHC ligands is the expansion of the carbenic heterocycle.6 Both of these properties are presumed to influence the reactivity of complexes by facilitating the formation of a catalytically active intermediate and increasing its stability. On the other hand, ring-expanded NHC (RE-NHC) may enable an intramolecular C–H activation, which can be detrimental to catalytic performance.7
Although RE-NHC nickel complexes are known, these studies are limited to only a few papers.7,8 To the best of our knowledge, to date there have been no reports on [Ni(Cp)(X)(RE-NHC)]. Driven by scientific curiosity to see if [Ni(Cp)(X)(RE-NHC)] complexes are of a greater catalytic activity than their congeners with five-membered “classical” NHCs, we decided to develop the synthesis of such complexes.
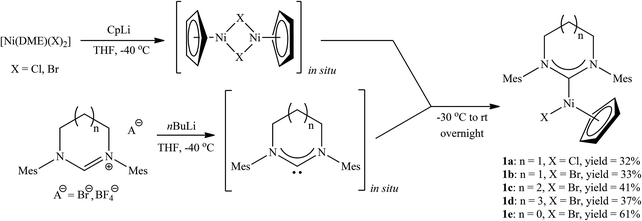
Our initial attempts to prepare the desired RE-NHC nickel complexes according to the procedure shown in Scheme 1a, i.e. from nickelocene and an appropriate carbene precursor, failed. Reactions involving the use of [Ni(acac)2] as the starting material were also unsuccessful (Scheme 1b). A probable reason for this lack of reactivity is the weak acidity of carbene precursors9 and steric congestion around the reacting centre.6 Inspired by the synthesis of related complexes with Cp*-functionalized NHC ligands,3g we decided to test a similar approach involving the addition of the two ligands (Cp and RE-NHC) to a nickel halide. Despite the plausible formation of different products, e.g. nickelocene and [Ni(X)2(RE-NHC)2], the desired [Ni(Cp)(X)(RE-NHC)] complexes were obtained in remarkably good yields as the only isolable products. More specifically, [Ni(DME)(X)2] (X = Br or Cl, DME = 1,2-dimethoxyethane) was treated first with a CpLi solution in THF to form what we believe is a dinuclear complex [Ni(Cp)(μ-X)]2.10 Subsequently, a freshly prepared RE-NHC solution, obtained by deprotonation of the carbene precursor with BuLi, was injected. After stirring overnight and conventional purification complexes 1a–1d were obtained in 32–41% yields (two steps from the Ni halide) as deep purple solids (Scheme 2).11
Moreover, to further expand the scope of our method, we have applied it to prepare a complex with a five-membered NHC. Thus, [Ni(Cp)(Br)(SIMes)]3c (SIMes = 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene) (1e) was obtained via this simple procedure in 61% yield (two steps from [Ni(DME)Br2]).
The NMR spectra of complexes 1a–1d were conventionally recorded in CDCl3 at ambient temperature. Signals originating from NHC ligands imply restricted Ni–Ccarbene and N–CMes bond rotation. Owing to the inequivalence of hydrogen atoms in each NCH2 group their signals appear in the 1H NMR spectra as unresolved multiplets. Moreover, as a result of the hindered rotation, all complexes 1a–1d have a characteristic set of signals for mesityl wingtips, showing two separated equal singlets (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) for meta hydrogens from phenyl rings and three singlets (6
2 ratio) for meta hydrogens from phenyl rings and three singlets (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) for methyl groups, i.e. ortho methyls and meta hydrogens are nonequivalent within each mesityl moiety. Another interesting feature of 1H NMR spectra concerns the Cp group. While for [Ni(Cp)(X)(NHC)] with five-membered NHCs the Cp singlet appears from 5.2 to 4.6 ppm, its chemical shift for 1a–1d is unusual and strongly influenced by the size of the NHC as it varies from 4.21 ppm for 1b to 3.68 ppm for 1d. Moreover, all signals in the 1H NMR spectrum of 1d are strongly broadened. We tentatively interpret these phenomena in terms of spin equilibrium,4f,12i.e. increasing content of the high-spin form of the complex which is correlated to the NHC's ring size.
6 ratio) for methyl groups, i.e. ortho methyls and meta hydrogens are nonequivalent within each mesityl moiety. Another interesting feature of 1H NMR spectra concerns the Cp group. While for [Ni(Cp)(X)(NHC)] with five-membered NHCs the Cp singlet appears from 5.2 to 4.6 ppm, its chemical shift for 1a–1d is unusual and strongly influenced by the size of the NHC as it varies from 4.21 ppm for 1b to 3.68 ppm for 1d. Moreover, all signals in the 1H NMR spectrum of 1d are strongly broadened. We tentatively interpret these phenomena in terms of spin equilibrium,4f,12i.e. increasing content of the high-spin form of the complex which is correlated to the NHC's ring size.
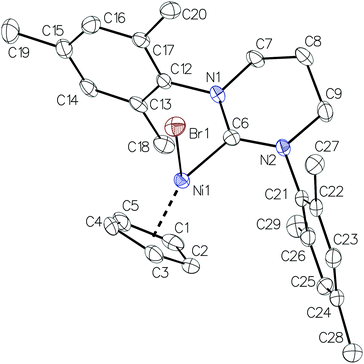
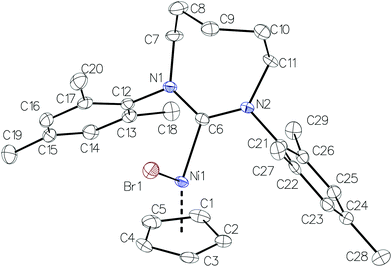
Crystal structure analysis of complexes 1b–1d revealed that they crystallize in the monoclinic crystal system and their molecular geometries are typical for nickel(II) NHC complexes (Fig. 1–3).13 The Ni–CCp bond lengths exhibit a pronounced trans influence on the RE-NHC ligands while the C–C bond lengths in Cp (see figure captions for selected bond lengths) show variations characteristic of the so-called “diene distortion”.14,15
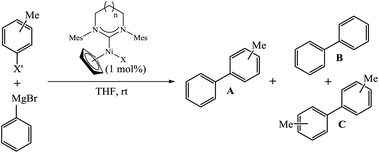
Complex [Ni(Cp)(Cl)(IMes)] (IMes = 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene) was reported as an active catalyst in the Kumada–Tamao–Corriu (KTC) coupling between p-tolylmagnesium bromide and phenyl O-sulfamate.3a To our surprise, further applications of [Ni(Cp)(X)(NHC)] complexes in this reaction are limited.3l,p,16 In order to get an insight into the influence of NHC ring expansion on the activity of [Ni(Cp)(X)(NHC)] in KTC coupling, we tested complexes 1a–1e in reactions of PhMgBr with halotoluenes (3-bromotoluene, 4-chlorotoluene). The expansion of the NHC ligand from a five- to six-membered ring proved to be beneficial for the catalytic activity (Table 1, entries 1–3, 9 and 10). A catalyst loading of only 1 mol% of 1a or 1b is sufficient to achieve conversions over 95% with very good selectivity at room temperature in 90 minutes.17 Also, the nature of the halogen ligand in the nickel complex appeared to have no influence on the catalytic performance (Table 1, entries 2 and 3). Further expansion of the NHC ligand to seven- or eight-membered rings resulted in diminished activity. However, high conversions could be achieved at longer reaction times (Table 1, entries 4–7). Selectivity is another parameter affected by ring expansion beyond the six-membered system (Table 1, entries 4, 5 and 7).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Catalyst | n | X′ | Conversionb (%) | Ratio A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cb Cb |
|---|---|---|---|---|---|
| a All reactions in duplicate; reaction conditions: THF (2.8 mL), halotoluene (0.66 mmol), phenylmagnesium bromide solution in THF (0.81 mL, 1.00 mmol, 1.24 mol L−1), nickel complex (6.7 μmol, 1 mol%), room temperature, 90 min; see the ESI for details. b Determined with GC. c Reaction time = 21 h. | |||||
| 1 | 1e | 0 | 3-Br | 78 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 2 | 1a | 1 | 3-Br | 97 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 3 | 1b | 1 | 3-Br | 98 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 1c | 2 | 3-Br | 36 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5c | 1c | 2 | 3-Br | 86 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 6 | 1d | 3 | 3-Br | Traces | — |
| 7c | 1d | 3 | 3-Br | 97 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8 | 1a | 1 | 4-Cl | Traces | — |
| 9c | 1a | 1 | 4-Cl | 68 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 10c | 1e | 0 | 4-Cl | 42 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Conclusions
We report here a new general synthesis of [Ni(Cp)(X)(NHC)] complexes that is applicable to ring-expanded NHC ligands. Complexes with a six-membered NHC provided the best efficiency in KTC coupling. Further studies will focus on the application of [Ni(Cp)(X)(RE-NHC)] in other catalytic reactions, including arylamination of aromatic halides.Acknowledgements
The authors thank the Warsaw University of Technology for financial support.Notes and references
- (a) C. D. Abernethy, A. H. Cowley and R. A. Jones, J. Organomet. Chem., 2000, 596, 3 CrossRef CAS; (b) R. A. Kelly III, N. M. Scott, S. Díez-González, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 3442 CrossRef.
- (a) V. Ritleng, C. Barth, E. Brenner, S. Milosevic and M. J. Chetcuti, Organometallics, 2008, 27, 4223 CrossRef CAS; (b) W. Buchowicz, J. Conder, D. Hryciuk and J. Zachara, J. Mol. Catal. A: Chem., 2014, 381, 16 CrossRef CAS.
- For catalytic applications of [Ni(Cp)(X)(NHC)], see: (a) T. K. Macklin and V. Snieckus, Org. Lett., 2005, 7, 2519 CrossRef CAS PubMed; (b) D. A. Malyshev, N. M. Scott, N. Marion, E. D. Stevens, V. P. Ananikov, I. P. Beletskaya and S. P. Nolan, Organometallics, 2006, 25, 4462 CrossRef CAS; (c) W. Buchowicz, A. Kozioł, L. B. Jerzykiewicz, T. Lis, S. Pasynkiewicz, A. Pęcherzewska and A. Pietrzykowski, J. Mol. Catal. A: Chem., 2006, 257, 118 CrossRef CAS; (d) W. Buchowicz, W. Wojtczak, A. Pietrzykowski, A. Lupa, L. B. Jerzykiewicz, A. Makal and K. Woźniak, Eur. J. Inorg. Chem., 2010, 648 CrossRef CAS; (e) V. Ritleng, A. M. Oertel and M. J. Chetcuti, Dalton Trans., 2010, 39, 8153 RSC; (f) A. M. Oertel, V. Ritleng and M. J. Chetcuti, Organometallics, 2012, 31, 2829 CrossRef CAS; (g) L. Postigo and B. Royo, Adv. Synth. Catal., 2012, 354, 2613 CrossRef CAS; (h) L. P. Bheeter, M. Henrion, L. Brelot, C. Darcel, M. J. Chetcuti, J.-B. Sortais and V. Ritleng, Adv. Synth. Catal., 2012, 354, 2619 CrossRef CAS; (i) A. R. Martin, Y. Makida, S. Meiries, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2013, 32, 6265 CrossRef CAS; (j) O. R. Luca, D. L. Huang, M. K. Takase and R. H. Crabtree, New J. Chem., 2013, 37, 3402 RSC; (k) L. P. Bheeter, M. Henrion, M. J. Chetcuti, C. Darcel, V. Ritleng and J.-B. Sortais, Catal. Sci. Technol., 2013, 3, 3111 RSC; (l) S. Tamba, K. Fuji, H. Meguro, S. Okamoto, T. Tendo, R. Komobuchi, A. Sugie, T. Nishino and A. Mori, Chem. Lett., 2013, 42, 281 CrossRef CAS; (m) M. Henrion, M. J. Chetcuti and V. Ritleng, Chem. Commun., 2014, 50, 4624 RSC; (n) A. Włodarska, A. Kozioł, M. Dranka, J. Jurkowski and A. Pietrzykowski, J. Mol. Catal. A: Chem., 2014, 395, 481 CrossRef; (o) Y. Wei, A. Petronilho, H. Mueller-Bunz and M. Albrecht, Organometallics, 2014, 33, 5834 CrossRef CAS; (p) P. Buchalski, R. Pacholski, K. Chodkiewicz, W. Buchowicz, K. Suwińska and A. Shkurenko, Dalton Trans., 2015, 44, 7169 RSC; (q) H. Valdés, M. Poyatos, G. Ujaque and E. Peris, Chem. – Eur. J., 2015, 21, 1578 CrossRef PubMed.
- For other examples of studies involving [Ni(Cp)(X)(NHC)], see: (a) E. F. Hahn, B. Heidrich, A. Hepp and T. Pape, J. Organomet. Chem., 2007, 692, 4630 CrossRef CAS; (b) C. Radloff, F. E. Hahn, T. Pape and R. Fröhlich, Dalton Trans., 2009, 7215 RSC; (c) A. M. Oertel, J. Freudenreich, J. Gein, V. Ritleng, L. F. Veiros and M. J. Chetcuti, Organometallics, 2011, 30, 3400 CrossRef CAS; (d) A. M. Oertel, V. Ritleng, L. Burr and M. J. Chetcuti, Organometallics, 2011, 30, 6685 CrossRef CAS; (e) A. M. Oertel, V. Ritleng, A. Busiah, L. F. Veiros and M. J. Chetcuti, Organometallics, 2011, 30, 6495 CrossRef CAS; (f) W. Buchowicz, Ł. Banach, J. Conder, P. A. Guńka, D. Kubicki and P. Buchalski, Dalton Trans., 2014, 43, 5847 RSC; (g) S. Pelties, D. Herrmann, B. de Bruin, F. Hartl and R. Wolf, Chem. Commun., 2014, 50, 7014 RSC.
- M. Henrion, A. M. Oertel, V. Ritleng and M. J. Chetcuti, Chem. Commun., 2013, 49, 6424 RSC.
- For selected examples, see: (a) P. Bazinet, G. P. A. Yap and D. S. Richeson, J. Am. Chem. Soc., 2003, 125, 13314 CrossRef CAS PubMed; (b) C. C. Scarborough, B. V. Popp, I. A. Guzei and S. S. Stahl, J. Organomet. Chem., 2005, 690, 6143 CrossRef CAS; (c) M. Iglesias, D. J. Beetstra, A. Stasch, P. N. Horton, M. B. Hursthouse, S. J. Coles, K. J. Cavell, A. Dervisi and I. A. Fallis, Organometallics, 2007, 26, 4800 CrossRef CAS; (d) M. Iglesias, D. J. Beetstra, J. C. Knight, L.-L. Ooi, A. Stasch, S. Coles, L. Male, M. B. Hursthouse, K. J. Cavell, A. Dervisi and I. A. Fallis, Organometallics, 2008, 27, 3279 CrossRef CAS; (e) M. Iglesias, D. J. Beetstra, B. Kariuki, K. J. Cavell, A. Dervisi and I. A. Fallis, Eur. J. Inorg. Chem., 2009, 1913 CrossRef CAS; (f) J. J. Dunsford and K. J. Cavell, Dalton Trans., 2011, 40, 9131 RSC; (g) J. J. Dunsford, K. J. Cavell and B. M. Kariuki, Organometallics, 2012, 31, 4118 CrossRef CAS; (h) E. L. Kolychev, A. F. Asachenko, P. B. Dzhevakov, A. A. Bush, V. V. Shuntikov, V. N. Khrustalev and M. S. Nechaev, Dalton Trans., 2013, 42, 6859 RSC; (i) O. S. Morozov, A. V. Lunchev, A. A. Bush, A. A. Tukov, A. F. Asachenko, V. N. Khrustalev, S. S. Zalesskiy, V. P. Ananikov and M. S. Nechaev, Chem. – Eur. J., 2014, 20, 6162 CrossRef CAS PubMed; (j) J. J. Dunsford and K. J. Cavell, Organometallics, 2014, 33, 2902 CrossRef CAS.
- C. J. E. Davies, M. J. Page, C. E. Ellul, M. F. Mahon and M. K. Whittlesey, Chem. Commun., 2010, 46, 5151 RSC.
- (a) P. D. Newman, K. J. Cavell and B. M. Kariuki, Organometallics, 2010, 29, 2724 CrossRef CAS; (b) R. C. Poulten, M. J. Page, A. G. Algarra, J. J. LeRoy, I. López, E. Carter, A. Llobet, S. A. Macgregor, M. F. Mahon, D. M. Murphy, M. Murugesu and M. K. Whittlesey, J. Am. Chem. Soc., 2013, 135, 13640 CrossRef CAS PubMed; (c) M. J. Page, W. Y. Lu, R. C. Poulten, E. Carter, A. G. Algarra, B. M. Kariuki, S. A. Macgregor, M. F. Mahon, K. J. Cavell, D. M. Murphy and M. K. Whittlesey, Chem. – Eur. J., 2013, 19, 2158 CrossRef CAS PubMed; (d) R. C. Poulten, I. López, A. Llobet, M. F. Mahon and M. K. Whittlesey, Inorg. Chem., 2014, 53, 7160 CrossRef CAS PubMed.
- E. M. Higgins, J. A. Sherwood, A. G. Lindsay, J. Armstrong, R. S. Massey, R. W. Alder and A. M. C. O'Donoghue, Chem. Commun., 2011, 47, 1559 RSC.
- Complexes of this type have been reported for multi-substituted Cp ligands, see: (a) U. Kölle, B. Fuss, F. Khouzami and J. Gersdorf, J. Organomet. Chem., 1985, 290, 77 CrossRef; (b) M. Schär, D. Saurenz, F. Zimmer, I. Schädlich, G. Wolmershäuser, S. Demeshko, F. Meyer, H. Sitzmann, O. M. Heigl and F. H. Köhler, Organometallics, 2013, 32, 6298 CrossRef.
- See the ESI† for details.
- M. E. Smith and R. A. Andersen, J. Am. Chem. Soc., 1996, 118, 11119 CrossRef CAS.
- The ESI† and CCDC 1429974–1429976 contain the supplementary crystallographic data for this paper.
- P. L. Holland, M. E. Smith, R. A. Andersen and R. G. Bergman, J. Am. Chem. Soc., 1997, 119, 12815 CrossRef CAS.
- Ł. Banach, P. A. Guńka, D. Górska, M. Podlewska, J. Zachara and W. Buchowicz, Eur. J. Inorg. Chem., 2015, 5677 CrossRef.
- A. E. Goetz and N. K. Garg, Nat. Chem., 2013, 5, 54 CrossRef CAS PubMed.
- We note that the activity of [Ni(Cp)(X)(RE-NHC)] in KTC coupling is comparable to that reported for the corresponding Pd complexes with standard five-membered NHCs, see: Z. Jin, X.-P. Gu, L.-L. Qiu, G.-P. Wu, H.-B. Song and J.-X. Fang, J. Organomet. Chem., 2011, 696, 859 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1429974–1429976. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt04663g |
| This journal is © The Royal Society of Chemistry 2016 |