Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Charge transfer complexes of fullerenes containing C60˙− and C70˙− radical anions with paramagnetic CoII(dppe)2Cl+ cations (dppe: 1,2-bis(diphenylphosphino)ethane)†
Dmitri V.
Konarev
*a,
Sergey I.
Troyanov
b,
Akihiro
Otsuka
c,
Hideki
Yamochi
c,
Gunzi
Saito
de and
Rimma N.
Lyubovskaya
a
aInstitute of Problems of Chemical Physics RAS, Chernogolovka, Moscow region, 142432 Russia. E-mail: konarev3@yandex.ru
bChemistry Department, Moscow State University, Leninskie Gory, 119991 Moscow, Russia
cResearch Center for Low Temperature and Materials Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
dFaculty of Agriculture, Meijo University, 1-501 Shiogamaguchi, Tempaku-ku, Nagoya 468-8502, Japan
eToyota Physical and Chemical Research Institute, 41-1, Yokomichi, Nagakute, Aichi 480-1192, Japan
First published on 19th February 2016
Abstract
The reduction of CoII(dppe)Cl2 with sodium fluorenone ketyl produces a red solution containing the CoI species. The dissolution of C60 in the obtained solution followed by the precipitation of crystals with hexane yields a salt {CoI(dppe)2+}(C60˙−)·2C6H4Cl2 and a novel complex {Co(dppe)2Cl}(C60) (1). With C70, only the crystals of {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) are formed. Complex 1 contains zig-zag fullerene chains whereas closely packed double chains are formed from fullerenes in 2. According to the optical spectra and magnetic data charge transfer occurs in both 1 and 2 with the formation of the CoII(dppe)2Cl+ cations and the C60˙− or C70˙− radical anions. In spite of the close packing in crystals, C60˙− or C70˙− retain their monomeric form at least down to 100 K. The effective magnetic moments of 1 and 2 of 1.98 and 2.27μB at 300 K, respectively, do not attain the value of 2.45μB expected for the system with two non-interacting S = 1/2 spins at full charge transfer to fullerenes. Most probably diamagnetic {CoI(dppe)2Cl}0 and neutral fullerenes are partially preserved in the samples which can explain the weak magnetic coupling of spins and the absence of fullerene dimerization in both complexes. The EPR spectra of 1 and 2 show asymmetric signals approximated by several lines with g-factors ranging from 2.0009 to 2.3325. These signals originate from the exchange interaction between the paramagnetic CoII(dppe)2Cl+ cations and the fullerene˙− radical anions.
Introduction
Ionic compounds of fullerenes possess promising conducting and magnetic properties.1–4 Crystalline samples of these compounds are generally prepared using organic or solvated metal cations.5,6 Metallocenes are strong donor molecules to give charge-transfer (CT) complexes composed of mono- to tri-anionic fullerenes.7–10 Also, by employing extra reducing agents such as alkali metals or tetrakis(dimethylamino)ethylene (TDAE), anionic fullerene complexes with organometallic cations are prepared. The latter method provided complexes with {(Ph3P)3AuI}+,11 CoI(dppe)2+ (ref. 12) and positively charged Ph3P-containing gold clusters,13 for example. The organometallic cations can introduce paramagnetic centers into the ionic fullerene systems.In our previous work we studied coordination compounds of fullerene C60 with cobalt in zero oxidation state containing Ph3P and diphosphine ligands (including 1,2-bis(diphenylphosphino)ethane, dppe) and in some cases benzonitrile.14–16 In this work we studied the interaction of fullerenes C60 and C70 with Co(dppe)2Cl prepared by the reduction of CoII(dppe)Cl2 with sodium fluorenone ketyl. Crystalline {Co(dppe)2Cl}(C60) (1) and {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) compounds were obtained together with the previously studied salt {CoI(dppe)2+}(C60˙−)·2C6H4Cl2.12 We present crystal structures, and optical and magnetic properties of these complexes. Compound 2 is a rare example of an ionic fullerene structure containing monomeric C70˙− radical anions while they form single-bonded (C70−)2 dimers17–19 in most of the anion radical salts.
Results and discussion
Synthesis
As summarized in Scheme 1, the first reduction potentials for fullerenes C60 and C70 are −0.44 V and −0.41 V, respectively, in dichloromethane vs. SCE. The second reduction wave is observed at −0.82 and −0.80 V vs. SCE, respectively, in the same solvent.20 The {CoII(dppe)2(CH3CN)}2+ cations can be reduced electrochemically to {CoI(dppe)2}+ at −0.70 V vs. Fc+/Fc (−0.275 V vs. SCE).21 Previously we used tetrakis(dimethylamino)-ethylene (TDAE) as the reductant for CoII(dppe)Br2 and C60.12 TDAE with the first oxidation potential of E+/0 = −0.75 V vs. SCE22 generates the CoI species and C60˙− in solution which cocrystallize to form the {CoI(dppe)2+}(C60˙−)·2C6H4Cl2 salt.12 While the CoI(dppe)2+ cation has an odd S = 1 or S = 0 spin state, only the C60˙− signal is observed in the EPR spectrum of this salt. Further reduction of CoI(dppe)2+ to Co0(dppe)2 by TDAE is not possible (Scheme 1) since the conversion takes place at a more negative redox potential of −1.56 V vs. Fc+/Fc (−1.135 V vs. SCE).21 The CoI(dppe)2+ cation is also too weak a donor to reduce C60˙− to C602−. | ||
| Scheme 1 Redox potentials of the components used in the synthesis of Co(dppe)-fullerene complexes. All potentials are given vs. SCE. | ||
Sodium fluorenone ketyl (fluorenone˙−)(Na+) is a stronger reducing agent than TDAE (Scheme 1) with the first oxidation potential of about −1.30 V vs. Ag/AgCl or (−1.255 V vs. SCE).23 Therefore, this ketyl can also reduce CoII(dppe)Cl2 to generate the CoI species. Potentially it can also reduce CoII(dppe)Cl2 to the Co0 species but such a redox process is hindered in nonpolar o-dichlorobenzene.
In this study, the formation of CoI species was detected by the color change of the reaction mixture from the green color of CoII(dppe)Cl2 to red during the reduction with sodium fluorenone ketyl. After the removal of unreacted sodium fluorenone ketyl by filtration, the generated CoI(dppe)n+ (n = 1, 2) was treated with neutral fullerenes. CoI(dppe)2Cl is rather a strong donor and potentially it can reduce fullerenes providing the CT complexes composed of CoII(dppe)22+ and C60˙− or C70˙− radical anions. While the main product of this reaction was {Co(dppe)2Cl}(C60) (1), the {CoI(dppe)2+}(C60˙−)·2C6H4Cl2 salt12 is also crystallized in 20% yield. The {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) complex crystallized exclusively with C70.
Optical properties
The IR spectra of 1 and 2 are listed in Table S1† and are shown in Fig. 1S and 2S.† The spectra practically seem to be the superposition of the absorption bands of Co(dppe)2Cl and fullerene anions C60˙− or C70˙−. Neutral fullerene C60 shows four F1u mode IR bands at 527, 576, 1183, and 1429 cm−1 (denoted as F1u(1) to F1u(4), respectively).5,24,25 While other bands exhibit only a few cm−1 shifts, the F1u(4) mode shows a 37 cm−1 red shift when C60 is charged −1. The strong absorption band observed at 1393 cm−1 for 1 unambiguously indicates the formation of C60˙−. The coexistence of partially reduced or neutral C60 cannot be confirmed from the IR spectrum since Co(dppe)2Cl has intense absorption bands at 1433 and 530 cm−1 which coincide with those of neutral C60. A similar situation is observed for complex 2 containing C70 (Table S1†).The UV-visible-NIR spectra of 1 and 2 are shown in Fig. 1. The absorption bands in the spectrum of 1 at 38000, 29700 cm−1 (262, 334 nm) and the weaker band at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 cm−1 (612 nm) can be attributed to C60 whereas the bands in the NIR range at 10
860 cm−1 (612 nm) can be attributed to C60 whereas the bands in the NIR range at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 and 9240 cm−1 (948, 1087 nm) (Fig. 1a) show the presence of C60˙−.5,6 The broad low-energy band of relatively weak intensity at about 7660 cm−1 (1300 nm) (Fig. 1a) can be ascribed to the CT transition between fullerenes or the Co(dppe)2Cl and fullerene species. Similarly, absorption bands in the spectrum of 2 at 26
650 and 9240 cm−1 (948, 1087 nm) (Fig. 1a) show the presence of C60˙−.5,6 The broad low-energy band of relatively weak intensity at about 7660 cm−1 (1300 nm) (Fig. 1a) can be ascribed to the CT transition between fullerenes or the Co(dppe)2Cl and fullerene species. Similarly, absorption bands in the spectrum of 2 at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 20
000 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 cm−1 (382 and 480 nm) are ascribed to C70. The broad absorption band in the NIR range can be reproduced by two Gaussian curves with maxima at about 9900 and 7500 cm−1 (1010 and 1330 nm, Fig. 1b and 2). The position of the latter band is close to that in the solution spectrum of monomeric C70˙−.5,6,26 It should be noted that generally C70 monoanions form singly bonded (C70−)2 dimers in solids to show two bands in the NIR range at about 11
840 cm−1 (382 and 480 nm) are ascribed to C70. The broad absorption band in the NIR range can be reproduced by two Gaussian curves with maxima at about 9900 and 7500 cm−1 (1010 and 1330 nm, Fig. 1b and 2). The position of the latter band is close to that in the solution spectrum of monomeric C70˙−.5,6,26 It should be noted that generally C70 monoanions form singly bonded (C70−)2 dimers in solids to show two bands in the NIR range at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 and 8200 cm−1 (880 and 1220 nm, Fig. 2).17–19 The strong shift of the (C70−)2 band at 880 nm to 1010 nm in the spectrum of 2 proves the failure of dimer formation in 2. So far, monomeric C70˙− are preserved only in the (Ph4P+)2(C70˙−)(I−) salt due to the long distances between fullerenes.27 The broad band at about 9900 cm−1 (1010 nm) can be attributed to the CT between fullerenes or the Co(dppe)2Cl and the fullerene species. Low energy CT bands (ca. 2000–5000 cm−1) which correspond to the Drude type reflectivity spectra characteristic of metals are not observed in the spectra of both 1 and 2.
360 and 8200 cm−1 (880 and 1220 nm, Fig. 2).17–19 The strong shift of the (C70−)2 band at 880 nm to 1010 nm in the spectrum of 2 proves the failure of dimer formation in 2. So far, monomeric C70˙− are preserved only in the (Ph4P+)2(C70˙−)(I−) salt due to the long distances between fullerenes.27 The broad band at about 9900 cm−1 (1010 nm) can be attributed to the CT between fullerenes or the Co(dppe)2Cl and the fullerene species. Low energy CT bands (ca. 2000–5000 cm−1) which correspond to the Drude type reflectivity spectra characteristic of metals are not observed in the spectra of both 1 and 2.
 | ||
| Fig. 1 Spectra of 1 (a) and 2 (b) in the UV-visible-NIR range measured at room temperature using KBr pellets prepared under anaerobic conditions. | ||
 | ||
| Fig. 2 The comparison of visible-NIR spectra of the C70˙− radical anions in 2 (upper spectrum) and the (C70−)2 dimers in the complex (Cp2Co+)2(C70−)2·2C6H4Cl2 (lower spectrum).8 | ||
Crystal structures
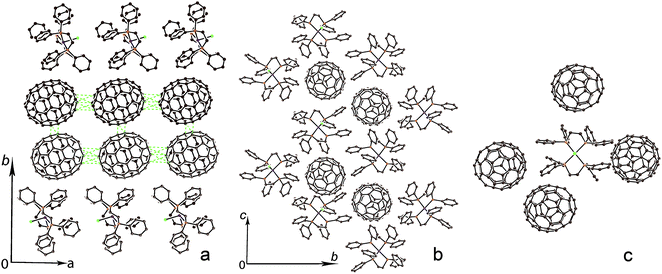
{Co(dppe)2Cl}(C60) (1) contains closely packed zigzag fullerene chains arranged along the c axis with equal interfullerene center-to-center (ctc) distances of 9.97 Å. This distance is noticeably shorter than the van der Waals (vdW) diameter of C60 (10.18 Å) and multiple vdW C⋯C contacts are formed between fullerenes (shown by green dashed lines in Fig. 3a). Fullerene chains are isolated (Fig. 3b), and the shortest ctc interfullerene distance between the neighboring chains is 12.53 Å.Each Co(dppe)2Cl unit is surrounded by four C60 cages (Fig. 3c). Nearly spherical C60 is inserted into the cavities formed by four phenyl substituents of Co(dppe)2Cl, and multiple vdW C,H(Co(dppe)2Cl)⋯C(C60) contacts are formed. One of the four surrounding fullerenes forms short Cl(Co(dppe)2Cl)⋯C(C60) contacts of the 2.998–3.107 Å length. The shortest distances (5.22–5.41 Å) between cobalt and the C60 carbon atoms are attained with the fullerene closest to the chloride anion of Co(dppe)2Cl.
Fullerenes C70 form closely packed double chains arranged along the a axis in {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2). The longer axis of C70 is directed along this axis, and the ctc interfullerene distance is 10.81 Å in this direction. The ctc interfullerene distance in the double chains along the b axis is 10.18 Å. As a result, multiple short vdW C⋯C contacts are formed between fullerenes (shown with green dashed lines in Fig. 4a). Double fullerene chains are completely isolated to give the ctc interfullerene distances among the neighboring double chains longer than 14 Å (Fig. 4b). Each Co(dppe)2Cl unit is surrounded by four C70 cages in 2 (Fig. 4c). In this case phenyl substituents cannot form a suitable cavity for a larger C70 ellipsoid, and it is positioned asymmetrically to Co(dppe)2Cl (Fig. 4c). There are no short Cl(Co(dppe)2Cl)⋯C(C70) contacts in 2 unlike the crystal structure of 1. The shortest Co⋯C(C70) distances are 5.94–6.70 Å.
The arrangement of ligands around cobalt atoms in the Co(dppe)2Cl units is similar in 1 and 2. However, these units have different bond lengths at the cobalt atoms. The cobalt atoms are located in the pyramidal environment in both the units formed by four phosphorus atoms and one chloride anion. The Co atoms are not located strictly in the plane of four phosphorus atoms but move out of this plane by 0.110 and 0.124 Å towards chloride anion in 1 and 2, respectively. The dimension of pyramidal coordination is informative to evaluate the charge on Co. As listed in Table 1, the average of Co–P length is increased according to the charge on Co (see, #0, 3–5).
| Compound | Co–Cl | Co–P (average) | Ref. | |
|---|---|---|---|---|
| #0 | {CoI(dppe)2}+(C60˙−)·2C6H4Cl2 (almost square planar) | 2.193(2) | 12 | |
| #1 | {Co(dppe)2Cl}(C60) (1) | 2.366(2) | 2.2495(8) | This work |
| #2 | {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) | 2.403(3) | 2.2725(8) | This work |
| #3 | {CoII(dppe)2Cl}+(SnCl3−)·C6H5Cl | 2.398(2) | 2.275(2) | 28 |
| #4 | {CoII(dppe)2I}+(I−)·CHCl3 | 2.283(3) | 29 | |
| #5 | {CoII(dppe)2(CH3CN)}2+ (BF4−)2·C3H6O | 2.306(3) | 21 |
The value of 2.2725(8) Å for 2 proves that Co is oxidized in this complex to almost +2, which corresponds well to a similar Co–Cl length to that of #3. On the contrary, the average Co–P length in 1 is shortened compared to those in CoII materials.
Compared with that in #0, the Co in 1 is regarded not to be oxidized fully to +2. This evaluation corresponds well to the optical spectra and magnetic data for 1.
Magnetic properties
Magnetic properties of 1 and 2 were studied by SQUID and EPR techniques. Effective magnetic moments of 1 and 2 are 1.98 and 2.27μB at 300 K, respectively. These values are intermediate between those characteristic of the systems of one and two non-interacting S = 1/2 spins per formula unit (1.73 and 2.45μB, respectively). In the case of 2, the magnetic moment is closer to 2.45μB than that of 1. We suppose that paramagnetic CoII(dppe)2Cl+ cations and C60˙− or C70˙− radical anions with the S = 1/2 spin state are formed in both complexes due to CT from Co(dppe)2Cl to fullerenes. Since the magnetic moment of 2.45μB is expected in the fully charge-transferred {CoII(dppe)2Cl}+(fullerene)˙− state, the observed lower magnetic moments are most probably due to the coexistence of diamagnetic {CoI(dppe)2Cl}0(fullerene)0 with the total magnetic moments of S = 0. Moreover, magnetic moments are not constant at high temperature and increases above 220 K for 1 and 140 K for 2 (Fig. 5a and b). Such behavior can be explained by the increase of the degree of CT from Co(dppe)2Cl to fullerenes with temperature. The low-temperature part can be approximated well by the Curie–Weiss expression with Weiss temperatures of only −2 and −3.5 K for 1 and 2, respectively (Fig. 5c and d). Thus, in spite of close packing of fullerenes in 1 and 2, only weak antiferromagnetic coupling of spins and no long range ordering are observed in both complexes. The presence of neutral diamagnetic components and disordered fullerenes can interrupt the phase transition. Incomplete CT to fullerenes can also be the reason for the absence of fullerene dimerization in spite of their close packing in a crystal. Particularly, complex 2 should be noted since the formation of stable singly bonded (C70−)2 dimers is observed in almost all the ionic compounds of C70.17–19 The similar exceptional absence of dimerization was found for C60 complexes with partial CT states on average.30 | ||
| Fig. 5 Temperature dependencies for effective magnetic moments of 1 (a) and 2 (b), and reciprocal molar magnetic susceptibility of 1 (c) and 2 (d). | ||
Complex 1 manifests an intense asymmetric EPR signal at room temperature which can be fitted well by three components with g1 = 2.0968 (the linewidth (ΔH) of 15.51 mT), g2 = 2.0488 and ΔH = 11.06 mT and g3 = 2.0085 and ΔH = 8.16 mT (Fig. 6a). The starting CoI has S = 1 or most probably S = 0 spin state. Compounds of CoI with the S = 1 spin state can manifest triplet EPR signals, for example, CoI(Ph3P)3X (X = Cl, Br),31 but these signals cannot be observed by X-band EPR spectroscopy. Only high-frequency and -field EPR spectroscopy can be used for these purposes. Neutral fullerenes are EPR silent. Therefore, only the paramagnetic CoII(dppe)2Cl+ and the C60˙− or C70˙− radical anions can contribute to the EPR signals of 1 and 2. Here, it is noted that the spin susceptibility estimated by the EPR signal intensity showed the same temperature dependency as that of magnetic susceptibility determined by the SQUID technique both for 1 and 2. This means that there is no contribution of CoI to the magnetism in these materials.
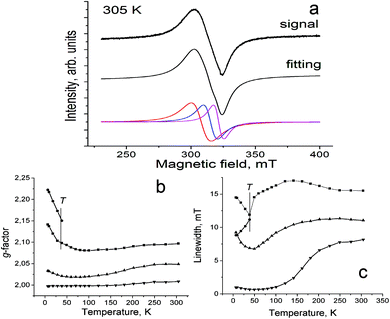 | ||
| Fig. 6 (a) EPR signal for polycrystalline 1 at 305 K; temperature dependence of components of the EPR signal: (b) g-factor, (c) linewidth. | ||
CoII(dppe)Br2 shows the Lorentzian EPR signal with g = 2.0357 and ΔH = 15.5 mT. However, CoII(dppe)Br2 has tetrahedral geometry while the CoII(dppe)2Cl+ cations have the pyramidal environment for the cobalt atoms. Essential modification of the cobalt environment in the formation of CoII(dppe)2Cl+ can affect the EPR signal by shifting g-factors to higher values in comparison with that of CoII(dppe)Br2. The signals with a g-factor closer to that of C60˙− are also observed (g2 = 2.0488 and g3 = 2.0085) together with the signals characteristic of CoII. These signals could be ascribed to both CoII(dppe)2Cl+ and C60˙− species having exchange interaction. They are strongly narrowed with the temperature decrease (Fig. 6c) and grow in intensity with the temperature increase above 220 K. We attribute such behavior to the increase of the CT degree from Co(dppe)2Cl to C60. The component with g3 = 2.0980 splits into two components below 40 K positioned at g = 2.0979 and 2.1501. Both components shift to higher g-factors with the temperature decrease (Fig. 6b).
Complex 2 shows an intense EPR signal at room temperature which can be fitted by three Lorentzian lines with g1 = 2.3325 (ΔH = 5.62 mT), g2 = 2.2649 (ΔH = 9.20 mT), and g3 = 2.1428 (ΔH = 24.84 mT) (Fig. 7). The broad g3-component splits into two lines below 220 K which are positioned at g = 2.1829 (ΔH = 13.86 mT) and 2.0711 (ΔH = 25.64 mT) at 220 K. Spectrum of 2 contains also weak narrow signal with g = 2.0021 (ΔH = 0.28 mT) (Fig. 7a) which can appear due to air oxidation of fullerene anions. It is seen that the EPR signal in 2 is broader and has essentially higher g-factors of the lines in comparison with those of 1. We can suppose that lines in the EPR spectrum of 2 with lower g-factors of 2.18 and 2.07 have essential contribution from C70˙−. It is known that the C70˙− radical anions in (Ph4P+)2(C70˙−)(I−) show a broad EPR signal with a g-factor of 2.0047 (ΔH = 60 mT) at 300 K.27 Therefore, the lines in the spectrum of 2 originating from both CoII(dppe)2Cl+ and C70˙− are essentially broader and shifted to higher g-factors in comparison with those of 1. Lines with lower g-factors (g = 2.18 and 2.07) slightly grow in intensity with the temperature increase above 150 K and that can also be explained by the increase of the CT degree from Co(dppe)2Cl to C70.
 | ||
| Fig. 7 EPR signal for polycrystalline 2 at 220 K (a); temperature dependence of the g-factor (b) and linewidth (c) of the components of the EPR signal of 2. | ||
Experimental
Materials
C60 of 99.9% purity and C70 of 99% purity were obtained from MTR Ltd and used without further purification. CoII(dppe)Cl2 (98%) was purchased from Aldrich. Sodium fluorenone ketyl was obtained as described.32 Solvents were purified under an argon atmosphere and degassed. o-Dichlorobenzene (C6H4Cl2) was distilled over CaH2 under reduced pressure and hexane was distilled over Na/benzophenone. All manipulations for the syntheses of 1 and 2 were carried out in an MBraun 150B-G glove box with a controlled argon atmosphere and the content of H2O and O2 less than 1 ppm. The solvents and crystals were stored in the glove box. Polycrystalline samples of 1 and 2 were placed in 2 mm quartz tubes under anaerobic conditions for EPR and SQUID measurements and sealed under 10−5 torr pressure. KBr pellets for IR- and UV-visible-NIR measurements were prepared in the glove box.Synthesis
Crystals of 1 and 2 were obtained by a diffusion technique. A reaction mixture was filtered into a 1.8-cm-diameter, 50 mL glass tube with a ground glass plug, and then 30 mL of hexane was layered over the solution. Slow mixing of the solutions resulted in precipitation of crystals over 1 month. The solvent was then decanted from the crystals, and they were washed with hexane. The compositions of the obtained salts were determined from X-ray diffraction analysis on a single crystal. Due to the high air sensitivity of 1 and 2, elemental analysis could not be carried out to determine the composition because the salts reacted with oxygen in air before the quantitative oxidation procedure.The crystals of {Co(dppe)2Cl}(C60) (1) and {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) were obtained by the following procedure. The reduction of green CoII(dppe)Cl2 (22 mg, 0.042 mmol) in 16 ml of C6H4Cl2 with sodium fluorenone ketyl (20 mg, 0.098 mmol) for 2 hours at 100 °C yielded a clear red solution. The solution was cooled down to room temperature and filtered into a flask containing fullerene C60 (30 mg, 0.042 mmol) for preparation of 1 and fullerene C70 (35 mg, 0.042 mmol) for preparation of 2. Fullerenes were dissolved in the obtained solution over 4 hours at 80 °C to produce violet-red and red solutions, respectively. After cooling down to room temperature the solutions were filtered into the tube for diffusion. The crystals of {Co(dppe)2Cl}(C60) (1) were obtained as black plates in 26% yield together with black elongated parallelepipeds of the previously reported salt {CoI(dppe)2+}(C60˙−)·2C6H4Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (yield is 20%) which was identified by X-ray diffraction on several parallelepiped-shaped single crystals. The crystals of two phases have different shapes and were separated under a microscope in the glove box. The purity of 1 was supported by the absence of the line at g = 1.9986 (ΔH = 4.57 mT) characteristic of {CoI(dppe)2+}(C60˙−)·2C6H4Cl2. The crystals of {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) were obtained as black plates in 42% yield. Testing of several crystals from the synthesis shows the presence of only one phase in this synthesis.
12 (yield is 20%) which was identified by X-ray diffraction on several parallelepiped-shaped single crystals. The crystals of two phases have different shapes and were separated under a microscope in the glove box. The purity of 1 was supported by the absence of the line at g = 1.9986 (ΔH = 4.57 mT) characteristic of {CoI(dppe)2+}(C60˙−)·2C6H4Cl2. The crystals of {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2) were obtained as black plates in 42% yield. Testing of several crystals from the synthesis shows the presence of only one phase in this synthesis.
General
UV-visible-NIR spectra were recorded using KBr pellets on a Perkin Elmer Lambda 1050 spectrometer in the 250–2500 nm range. FT-IR spectra were obtained using KBr pellets with a Perkin-Elmer Spectrum 400 spectrometer (400–7800 cm−1). EPR spectra were recorded for polycrystalline samples of 1 and 2 with a JEOL JES-TE 200 X-band ESR spectrometer equipped with a JEOL ES-CT470 cryostat working between room and liquid helium temperatures. A Quantum Design MPMS-XL SQUID magnetometer was used to measure the static magnetic susceptibility of 1 and 2 at 100 mT magnetic field under cooling and heating conditions in the 300–1.9 K range. The sample holder contribution and core temperature independent diamagnetic susceptibility (χd) were subtracted from the experimental values. The χd values were estimated by the extrapolation of the data in the high-temperature range by fitting the data with the following expression: χM = C/(T − Θ) + χd, where C is the Curie constant and Θ is the Weiss temperature. The effective magnetic moment (μeff) was calculated with the formula μeff = (8χMT)1/2.Crystal structure determination
The intensity data for 1 and 2 were collected on an IPDS (Stoe) diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). The structures were solved by a direct method and refined by a full-matrix least-squares method against F2 using SHELXL 2014/7.33 All non-hydrogen atoms were refined anisotropically. Positions of hydrogen atoms were included into refinement in a riding model. See the ESI† for crystallographic data in CIF format.Crystal data of 1 at 100(2) K: C112H48ClCoP4, Mr = 1611.76 g mol−1, black plate, monoclinic, C2/c, a = 22.1120(6), b = 19.1090(6), c = 17.0090(4) Å, β = 107.480(2)°, V = 6855.1(3) Å3, Z = 4, dcalc = 1.562 g cm−3, μ = 0.446 mm−1, F(000) = 3296, 2θmax = 58.4°, reflections measured 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820, unique reflections 9002, reflections with I > 2σ(I) = 6591, parameters refined 630, restraints 180, R1 = 0.0735, wR2 = 0.1851, G.O.F. = 1.083. The C60 cage is disordered over two orientations linked by a two-fold axis. CCDC 1437134.
820, unique reflections 9002, reflections with I > 2σ(I) = 6591, parameters refined 630, restraints 180, R1 = 0.0735, wR2 = 0.1851, G.O.F. = 1.083. The C60 cage is disordered over two orientations linked by a two-fold axis. CCDC 1437134.
Crystal data of 2 at 100(2) K: C125H50Cl2CoP4, Mr = 1805.36 g mol−1, black plate, monoclinic, P21/n, a = 10.8104(3), b = 39.8917(10), c = 18.2142(5) Å, β = 100.133(2)°, V = 7732.3(4) Å3, Z = 4, dcalc = 1.551 g cm−3, μ = 0.438 mm−1, F(000) = 3684, max. 2θmax = 53.5°, reflections measured 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143, unique reflections 15
143, unique reflections 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869, reflections with I > 2σ(I) = 10
869, reflections with I > 2σ(I) = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991, parameters refined 821, restraints 24, R1 = 0.0814, wR2 = 0.2035, G.O.F. = 1.000. The C70 cage is disordered between three orientations with the 0.45/0.30/0.25 occupancies. The solvent C6H4Cl2 molecule is statistically disordered between two orientations. CCDC 1437135.
991, parameters refined 821, restraints 24, R1 = 0.0814, wR2 = 0.2035, G.O.F. = 1.000. The C70 cage is disordered between three orientations with the 0.45/0.30/0.25 occupancies. The solvent C6H4Cl2 molecule is statistically disordered between two orientations. CCDC 1437135.
Conclusions
The interaction of the CoI species generated by the reduction of CoIIdppeCl2 allows the preparation of CT complexes {Co(dppe)2Cl}(C60) (1) and {Co(dppe)2Cl}(C70)·0.5C6H4Cl2 (2). Both complexes contain the C60˙− or C70˙− radical anions and the CoII(dppe)2Cl+ cations formed as a result of CT from CoI(dppe)2Cl to fullerenes. CT becomes possible due to the strong donor properties of CoI(dppe)2Cl which are enough to produce fullerene˙− radical anions. However, most probably CT is not complete and diamagnetic {CoI(dppe)2Cl}0 and neutral fullerenes are also preserved in the samples. As a result, in spite of the close packing of fullerenes in the chains only weak magnetic coupling of spins is observed and fullerenes are not dimerized in both complexes. The absence of dimerization allows for the first time to observe the solid state optical spectrum of monomeric C70˙− radical anions and to determine their molecular structure. It is also seen that organometallic compounds with strong donor properties can be promising components to design fullerene complexes with partial CT.Acknowledgements
The work was supported by the Russian Science Foundation (project no. 14-13-00028) and by JSPS KAKENHI Grant Numbers 23225005 and 26288035.Notes and references
- K. Tanigaki and K. Prassides, J. Mater. Chem., 1995, 5, 1515 RSC.
- P. W. Stephens, G. Bortel, G. Faigel, M. Tegze, A. Jánossy, S. Pekker, G. Oszlanyi and L. Forró, Nature, 1994, 370, 636 CrossRef CAS.
- D. V. Konarev, S. S. Khasanov, A. Otsuka, M. Maesato, G. Saito and R. N. Lyubovskaya, Angew. Chem., Int. Ed., 2010, 49, 4829 CrossRef CAS PubMed.
- P. W. Stephens, D. Cox, J. W. Lauher, L. Mihaly, J. B. Wiley, P.-M. Allemand, A. Hirsch, K. Holczer, Q. Li, J. D. Thompson and F. Wudl, Nature, 1992, 355, 331 CrossRef CAS.
- D. V. Konarev and R. N. Lyubovskaya, Russ. Chem. Rev., 2012, 81, 336 CrossRef CAS.
- C. A. Reed and R. D. Bolskar, Chem. Rev., 2000, 100, 1075 CrossRef CAS PubMed.
- W. C. Wan, X. Liu, G. M. Sweeney and W. E. Broderick, J. Am. Chem. Soc., 1995, 117, 9580 CrossRef CAS.
- D. V. Konarev, S. S. Khasanov, G. Saito, A. Otsuka, Y. Yoshida and R. N. Lyubovskaya, J. Am. Chem. Soc., 2003, 125, 10074 CrossRef CAS PubMed.
- D. V. Konarev, S. S. Khasanov, I. I. Vorontsov, G. Saito, Yu. M. Antipin and R. N. Lyubovskaya, Inorg. Chem., 2003, 42, 3706 CrossRef CAS PubMed.
- C. Bossard, S. Rigaut, D. Astruc, M.-H. Delville, G. Félix, A. Février-Bouvier, J. Amiell, S. Flandrois and P. Delhaès, J. Chem. Soc., Chem. Commun., 1993, 333 RSC.
- D. V. Konarev, S. S. Khasanov, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Inorg. Chem., 2014, 53, 6850 CrossRef CAS PubMed.
- D. V. Konarev, A. V. Kuzmin, S. V. Simonov, S. S. Khasanov, E. I. Yudanova and R. N. Lyubovskaya, Dalton Trans., 2011, 40, 4453 RSC.
- M. Schulz-Dobrick and M. Jansen, Angew. Chem., Int. Ed., 2008, 47, 2256 CrossRef CAS PubMed.
- D. V. Konarev, S. S. Khasanov, S. I. Troyanov, Y. Nakano, K. A. Ustimenko, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Inorg. Chem., 2013, 52, 13934 CrossRef CAS PubMed.
- D. V. Konarev, S. I. Troyanov, Y. Nakano, K. A. Ustimenko, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Organomet., 2013, 32, 4038 CrossRef CAS.
- D. V. Konarev, S. I. Troyanov, K. A. Ustimenko, Y. Nakano, A. F. Shestakov, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Inorg. Chem., 2015, 54, 4597 CrossRef CAS PubMed.
- D. V. Konarev, S. S. Khasanov, I. I. Vorontsov, G. Saito, Yu. A. Antipin, A. Otsuka and R. N. Lyubovskaya, Chem. Commun., 2002, 2548 RSC.
- D. V. Konarev, S. S. Khasanov, S. V. Simonov, E. I. Yudanova and R. N. Lyubovskaya, CrystEngComm, 2010, 12, 3542 RSC.
- D. V. Konarev, A. V. Kuzmin, S. V. Simonov, S. S. Khasanov, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Dalton Trans., 2012, 41, 13841 RSC.
- D. Dubois, K. M. Kadish, S. Flanagan, R. F. Haufler, L. P. F. Chibante and L. J. Wilson, J. Am. Chem. Soc., 1991, 113, 4364 CrossRef CAS.
- R. Ciancanelli, B. C. Noll, D. L. DuBois and M. R. DuBios, J. Am. Chem. Soc., 2002, 124, 2984 CrossRef CAS PubMed.
- K. Kuwata and D. H. Geske, J. Am. Chem. Soc., 1964, 86, 2101 CrossRef CAS.
- R. O. Loutfyc, K. Hsiaob, S. Ong and B. Keoshkeria, Can. J. Chem., 1984, 62, 1877 CrossRef.
- T. Picher, R. Winkler and H. Kuzmany, Phys. Rev. B: Condens. Matter, 1994, 49, 15879 CrossRef.
- V. N. Semkin, N. G. Spitsina, S. Krol and A. Graja, Chem. Phys. Lett., 1996, 256, 616 CrossRef CAS.
- D. V. Konarev, N. V. Drichko and A. Graja, J. Chim. Phys., 1998, 95, 2143 CrossRef CAS.
- A. Penicaud, A. P. Perez-Benitez, R. Escudero and C. Coulon, Solid State Commun., 1995, 96, 147 CrossRef CAS.
- J. K. Stalick, P. W. R. Corfield and D. W. Meek, Inorg. Chem., 1973, 12, 1668 CrossRef CAS.
- S. Aizawa, K. Fukumoto and T. Kawamoto, Polyhedron, 2013, 62, 37 CrossRef CAS.
- D. V. Konarev, S. S. Khasanov, M. Ishikawa, E. I. Yudanova, A. F. Shevchun, M. S. Mikhailov, P. A. Stuzhin, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Chem. Select, 2016, 1, 323 Search PubMed.
- J. Krzystek, A. Ozarowski, S. A. Zvyagin and J. Telser, Inorg. Chem., 2012, 51, 4954 CrossRef CAS PubMed.
- D. V. Konarev, S. S. Khasanov, E. I. Yudanova and R. N. Lyubovskaya, Eur. J. Inorg. Chem., 2011, 816 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: IR spectra of 1 and 2. CCDC 1437134 and 1437135. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt04627k |
| This journal is © The Royal Society of Chemistry 2016 |