Synthesis and reactivity of fluorenyl-tethered N-heterocyclic stannylenes†
Abstract
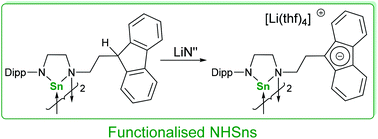
A fluorenyl (Fl) tethered diamine was synthesised by nucleophilic substitution of (bromoethyl)fluorene with a diisopropylphenyl (Dipp) substituted diamine to give FlC2H4N(H)C2H4N(H)Dipp (1a) in good yield (85%). Lithiation of 1a with n-BuLi proceeded with coordination of the Li cation to the aromatic fluorenide ring (2), and with subsequent equivalents of n-BuLi, the secondary amines were then sequentially deprotonated. A fluorenyl-tethered N-heterocyclic stannylene (NHSn) was synthesised from the reaction of 1a with SnN′′2 {N′′ = N(SiMe3)2} as a neutral dimeric species (5), and this was deprotonated with LiN′′ to give the corresponding dianionic fluorenide-tethered NHSn (6). Reactions of [{Rh(cod)(μ-Cl)}2] with the mono-deprotonated ligand 2 led to the formation of a mixed-donor amide–amine Rh(I) compound (7), whereas reactions with the anionic NHSn 6 led to a Rh–fluorenyl complex of low stability with an uncoordinated pendent NHSn arm, which X-ray crystallography showed to be dimeric in the solid state.
- This article is part of the themed collection: Main Group Transformations


 Please wait while we load your content...
Please wait while we load your content...