 Open Access Article
Open Access ArticleA comprehensive approach to investigate the structural and surface properties of activated carbons and related Pd-based catalysts†
A.
Lazzarini
a,
A.
Piovano
*b,
R.
Pellegrini
c,
G.
Leofanti
d,
G.
Agostini
e,
S.
Rudić
f,
M. R.
Chierotti
 a,
R.
Gobetto
a,
A.
Battiato
g,
G.
Spoto
a,
A.
Zecchina
a,
C.
Lamberti
ah and
E.
Groppo
*a
a,
R.
Gobetto
a,
A.
Battiato
g,
G.
Spoto
a,
A.
Zecchina
a,
C.
Lamberti
ah and
E.
Groppo
*a
aDepartment of Chemistry, NIS Centre and INSTM, University of Turin, Via Giuria 7, Turin, I-10125, Italy. E-mail: elena.groppo@unito.it
bInstitut Laue-Langevin (ILL), 71 avenue des Martyrs, 38000 Grenoble, France. E-mail: piovano@ill.fr
cChimet SpA - Catalyst Division, Via di Pescaiola 74, Viciomaggio Arezzo, I-52041 Italy
dConsultant, Via Firenze 43, 20010 Canegrate, Milano, Italy
eEuropean Synchrotron Radiation Facility (ESRF), 71 avenue des Martyrs, 38000 Grenoble, France
fISIS Facility, Rutherford Appleton Laboratory, Chilton, Didcot, Oxfordshire OX11 0QX, UK
gDepartment of Physics, NIS Centre, University of Turin, Via Giuria 1, Turin, I-10125, Italy
hSouthern Federal University, Zorge street 5, 344090 Rostov-on-Don, Russia
First published on 23rd February 2016
Abstract
Activated carbons are widely used as supports for industrial catalysts based on metal nanoparticles. The catalytic performance of carbon-supported catalysts is strongly influenced by the carbon activation method. Notwithstanding this important role, the effect induced by different activation methods has been rarely investigated in detail. This work deals with two carbons of wood origin, activated either by steam or by phosphoric acid, and the corresponding catalysts based on supported Pd nanoparticles. We demonstrate that the catalysts perform in a different way in hydrogenation reactions depending on the nature of the carbon used as a support, being the palladium dispersion the same. We propose a multi-technique approach to fully characterize both carbons and catalysts at the micro- and nanoscale. In particular, we investigate how the activation procedure influences the texture (by N2 physisorption), the morphology (by Scanning Electron Microscopy), the structure (by Solid State Nuclear Magnetic Resonance, Raman spectroscopy and X-ray Diffraction) and the surface properties (by X-ray Photoelectron Spectroscopy, Diffuse Reflectance Infrared Spectroscopy and Inelastic Neutron Scattering) of carbons and of the related catalysts. The comprehensive characterization approach proposed in this work allows the rationalization, at least in part, of the role of activated carbons in enhancing the performance of a hydrogenation catalyst.
1. Introduction
Activated carbons are important modifications of carbon that find a wide use in the field of catalysis, where they are usually employed as supports for noble metal nanoparticles. They consist of carbonized bio-polymeric materials activated in a second step of the synthesis process.1–3 A large number of patents describe numerous ways to activate carbon from several natural sources, such as wood, peat or coconut shells.1–7 Activation is usually performed in the presence of steam or by adding phosphoric acid to the raw product, resulting in activated carbons having different properties in terms of porosity, structure at a micro- and nanoscale, and surface chemistry. All these three factors play a fundamental role in catalysis. The support may have a direct influence on the catalytic reaction because its surface is often active toward reactants and reaction products.8 In addition, the support may exhibit an indirect influence because its physical–chemical properties may affect the properties of the deposited metal nanoparticles (such as shape, size distribution and dispersion), their tendency to aggregate under catalytic conditions (i.e. resistance to sintering), and the accessibility of active sites to reactants.1–3,9–13Pore size and pore volume are important factors for physical adsorption. Many works in the literature report detailed analysis of the porosity of carbons activated following different routes.5,7,14 It is now well established that in all cases a high specific surface area is obtained (up to 1500 m2 g−1) due to the oxidative generation of micropores of variable size and shape distribution. In the presence of phosphoric acid, a fraction of the additive is incorporated during carbonization into the carbon body and is subsequently removed by leaching; as a consequence, this procedure generally creates pores having a narrower pore size distribution with respect to those obtained by oxidation in steam.15 In terms of local structure, an activated carbon is usually described as composed of significant amounts of graphite-like (or sp2) species depending on the temperature of activation.16 Although all of them are characterized by the same predominant structural unit, activated carbons may differ in terms of their nano-structure, which means the connectivity of the sp2 domains from molecular dimensions up to a few nanometers. Finally, the surface chemistry of carbons plays a key role in specific adsorption and is relevant to all aspects of catalysis. The two most important hetero-elements are hydrogen and oxygen, each of which can undergo a variety of chemically different coordination geometries, creating a rich surface chemistry. Hydrogen is naturally present in activated carbons to terminate sp2 domains. In addition, it is well documented that a large number of oxygen functional groups are created during the activation process by the saturation of dangling bonds with oxygen.17,18
It is evident that activated carbons as catalyst supports (but also as catalysts on their own) offer incomparable flexibility in tailoring catalyst properties to specific needs. However, a comprehensive characterization of the structural and surface properties of activated carbons is fundamental to understanding their role in catalysis. Activated carbons have been widely investigated in the literature.1–3 Conflicting information is often found, along with highly fragmented results, mainly as a consequence of an uncritical use of different methodologies. Due to the intrinsic complexity of activated carbons, no single technique is able to give a complete picture of the overall properties of activated carbons; instead, a range of complementary characterization techniques is needed to characterise these materials at different dimension scales.
In this work we investigate in detail two activated carbons and the corresponding Pd/C catalysts. The two carbons originate from the same raw material (wood) but have been activated either by steam (CW) or by phosphoric acid (CChemi). At first, we explore the catalytic performance of Pd/C catalysts in hydrogenation reactions as a function of the activated carbon used as a support. Then, we systematically characterize the morphological, structural and surface properties of the two activated carbons and of the related catalysts by means of a multi-technique approach. In particular, we apply N2 physisorption to evaluate surface area and porosity, Scanning Electron Microscopy (SEM) to investigate the morphology at a micrometric scale, X-ray Powder Diffraction (XRPD), 1H and 13C Solid-State Nuclear Magnetic Resonance (SSNMR) and Raman Spectroscopy to determine the structural properties at the nanometric scale, X-ray Photoelectron Spectroscopy (XPS), Diffuse Reflectance Infrared Fourier-Transform (DRIFT) spectroscopy and Inelastic Neutron Scattering (INS) to investigate the surface properties. Although some of these techniques are commonly applied to investigate carbon-based materials, to the best of our knowledge this is the first time that such a large number of characterization techniques are simultaneously used to investigate the same activated carbons and related catalysts. In particular, to the best of our knowledge, no other work reports the synergic combination of Raman, DRIFT and INS spectroscopies to achieve a full vibrational characterization of carbon-based materials.
2. Experimental section
2.1 Materials
Two commercial grades of activated carbons, both having wood origin, were provided by Chimet S.p.A.19 CW is activated at high temperature in the presence of steam, whereas CChemi is activated at high temperature after impregnation with phosphoric acid.Pd/C catalysts (metal loading of 5.0 wt%) were prepared in the Chimet Laboratories on CW and CChemi carbons, following the deposition–precipitation method as reported elsewhere.9 Na2PdCl4 was used as the palladium precursor and Na2CO3 as the basic agent. All the catalysts were water-washed until residual chlorides were removed and dried at 120 °C overnight. No metal was found in the solution after filtration (as determined by ICP). In some cases, pre-reduction was carried out before washing with formate at 65 °C for 1 h.20 Hereafter, Pd/CW and Pd/CChemi will be used to indicate the unreduced Pd-based catalysts prepared on CW and CChemi carbons, respectively. When a pre-reduction is performed, a label indicating the reducing agent is added. Thus, Pd/Cw(F) and Pd/CChemi(F) refer, respectively, to CW and CChemi supported palladium catalysts (5.0 wt% loading) reduced with formate.
2.2 Catalytic performance in hydrogenation reactions
The catalytic performance of the Pd/C catalysts was tested in a transfer hydrogenation21 and a debenzylation reaction.22 Transfer hydrogenation of resorcinol to 1,3-cyclohexanedione23 was carried out in a 300 cm3 glass reactor equipped with a double mantle for water circulation, a magnetic stirrer, a gas inlet and a reflux condenser. Water at the required temperature was circulated inside the reactor mantle by means of a circulation bath. The reactor was charged with 125 ml of water, 55.0 g of resorcinol and 44.2 g of sodium formate. The reaction mixture was heated to 40 °C while stirring and purging the reaction medium with nitrogen gas for 30 minutes. Then, 2.75 g of Pd/C catalyst were added and held for 3 hours. After that, the temperature was raised to 55 °C and maintained for 15 hours. Samples were withdrawn at 2, 3, and 18 hours and analysed by HPLC to determine conversion and selectivity.Debenzylation of N-benzyl-N-ethylaniline to N-ethylaniline and toluene24 was carried out in a 500 cm3 autoclave equipped with a heating mantle, mechanical stirrer and gas regulation system able to perform the reaction at constant pressure. The autoclave was charged with 300 ml of ethanol, 150 g of N-benzyl-N-ethylaniline and 3.0 g of Pd/C catalyst. The autoclave was closed and purged first with nitrogen and then with hydrogen. Hydrogenation was performed at a temperature of 65 °C, a pressure of 3 bar and a stirring speed of 900 rpm. The automatic recording of the hydrogen consumption allowed plotting of the consumption curve and then the catalytic activity expressed in mmolH2min−1 gcat−1.
2.3 Methods
The XPS analysis depth in the case of carbon matrix is about 10–15 nm. The spectra were analysed and processed with the use of Unifit2008© software. The background was approximated by a third-order polynomial function combined with the Shirley model for inelastic processes, and the detailed spectra were fitted with a convolution of Gaussian functions.
3. Results and discussion
3.1 Catalytic performance of Pd/C catalysts in hydrogenation reactions
The catalytic performance of the Pd/C catalysts in two selective hydrogenation reactions is summarized in Tables 1 and 2. The pre-reduced Pd/CW(F) and Pd/CChemi(F) catalysts were tested in the transfer hydrogenation of resorcinol to 1,3-cyclohexanedione using sodium formate as the hydrogen source (Chart 1 and Table 1). Although the two catalysts have a similar metal dispersion and display a similar conversion as a function of time, they give a different selectivity to 1,3-cyclohexanedione. In particular, Pd/CW(F) is more selective than Pd/CChemi(F), reaching almost 100% selectivity at full conversion.| Catalyst | D (%) | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
| 2 h | 3 h | 18 h | |||
| Pd/CW(F) | 28.1 | 30.8 | 40.2 | 96.2 | 98.82 |
| Pd/CChemi(F) | 26.3 | 29.4 | 38.1 | 96.8 | 85.16 |
| Catalyst | D (%) | Activity (mmolH2min−1 gcat−1) |
|---|---|---|
| Pd/CW | 23.5 | 14.6 |
| Pd/CChemi | 18.8 | 31.1 |
The unreduced Pd/CW and Pd/CChemi catalysts were tested in the debenzylation of N-benzyl-N-ethylaniline to give N-ethylaniline and toluene (Chart 2 and Table 2). Also in this case the metal dispersion is quite similar for the two catalysts, but Pd/CChemi is much more active than Pd/CW.
These data clearly demonstrate that the catalytic performance (in terms of both activity and selectivity) of Pd/C catalysts in hydrogenation reactions is strongly affected by the nature of the support, being the palladium dispersion similar in the two couples of catalysts. This is well known in industrial practice, but the reasons were never investigated in a systematic way. In most of the cases only the metal phase was characterized in detail, whereas very little attention was devoted to the investigation of the support properties, especially when dealing with carbons whose “black” nature makes the characterization techniques using IR, visible and near-UV photons not straightforwardly applicable.31–33
3.2 Chemical composition, morphology and porous texture of CW and CChemi
The average composition of CW and CChemi was determined by means of Electron Dispersive X-ray (EDX) analysis (Table S1†) by averaging the measurements performed on five different carbon granules. Complementary information on the surface composition was obtained by XPS spectroscopy (Fig. S1, Tables S2 and S3†). Both carbons contain a substantial amount of oxygen, which is at least in part contained in inorganic insoluble ashes. The ash content in the two carbons was quantitatively determined by calcination and resulted in 0.58 wt% for CW and 1.96 wt% for CChemi. Compositional analysis indicates that they are mainly composed of silico-aluminates. Accordingly, Si is detected by XPS in CChemi and hardly visible in CW. Phosphorus is also present in the inorganic ashes in CChemi (see below). The role of the inorganic ashes in affecting the catalysts' performance is expected to be marginal because of their very low concentration.In addition, CChemi carbon contains a consistent amount of phosphorus, in agreement with recent literature reporting that activation with phosphoric acid not only develops porosity but also leads to the inclusion of a significant amount of phosphorus into the carbon structure.34–37 The nature and localization of the phosphorus contained in CChemi was clarified by 31P Cross-Polarization Magic Angle Spinning (CP-MAS) Solid-State Nuclear Magnetic Resonance (SSNMR) measurements and X-ray photoelectron spectroscopy. The 31P CPMAS NMR spectrum (Fig. S2†) is characterized by two main signals centred around 1.3 and 14.1 ppm. The former is characteristic of phosphates, i.e. phosphorus bound to four oxygen atoms, likely contained in the inorganic ashes. On the contrary, the resonance at 14.1 ppm is attributed to phosphonates, i.e. organo-phosphorus compounds containing one P–C bond, revealing that at least a fraction of phosphorus has functionalized the carbon.34–37 Interestingly, XPS measurements (Fig. S1†) do not reveal the presence of phosphorus, indicating that it is not localized at the surface of CChemi, and hence it is not relevant for catalysis. This is in contrast to the findings of Puziy et al.,37 which, however, are related to chemically activated carbons of different origin (polymer- and fruit-stone-based carbons).
Fig. 1 shows a few representative SEM picture of CW (a) and CChemi (b) carbons. In both cases, irregular micro-particles are observed, which resemble the peculiar structures of the pristine wood. In particular, reminiscence of the original vascular bundles and tracheids are clearly observed in CW (see inset in Fig. 1a), whereas they are hardly detectable for CChemi, which shows on average much smaller micro-particles. These morphological differences on a microscale are in good agreement with the average particle size as determined by light scattering, and reflect the harsher activation conditions for CChemi. It is expected that the different micro-structure has an influence on the macroscopic mechanical properties of the two carbons. Finally, the textural properties of the two carbons were investigated by means of N2 physisorption at 77 K. Fig. 1c shows the N2 physisorption isotherms collected at 77 K for CW and CChemi carbons. The activation process substantially affects the textural properties of carbons. The specific surface area is approximately 1000 m2 g−1 for the CW and 1500 m2 g−1 for CChemi, which also displays a significantly larger total pore volume.
 | ||
| Fig. 1 Representative SEM images of CW (a) and CChemi (b). (c) N2 physisorption isotherms collected at 77 K for CW (grey) and CChemi (white). | ||
3.3 Structural properties of CW and CChemi at a nanometric scale
 | ||
| Fig. 2 XRPD patterns of CW (grey) and CChemi (black) carbons after subtraction of the background and assignment of the main peaks (λ = 1.5409 Å). The inset shows the patterns as collected. | ||
The lateral size (La) of the sp2-ordered crystallites has been estimated from the width of the (100) and (111) peaks by using the Scherrer equation, resulting in La = 16.5 ± 0.5 Å for CW and La = 12.5 ± 0.5 Å for CChemi. However, it should be noted that several discrepancies are reported in the literature on the exact determination of the average crystallite size according to the different methods of peak profile evaluation and also that defects and strain within the carbon lattice would contribute to the diffraction broadening.16,42–44 For the present discussion, it is sufficient to note that the activation procedure influences the in-plane dimension of the graphitic domains in carbons. In particular, CW is characterized, on average, by sp2 crystallites larger than CChemi, but in both cases the lateral size of regular sp2 islands is smaller than 2 nm. It is worth noting that the presence of defects (such as heteroatoms) is sufficient to remove the aromaticity and hence to disrupt the regularity of the sp2 domains.
The 13C CPMAS NMR spectra of both CW and CChemi carbons, shown in Fig. 3, are entirely dominated by a broad peak centered around 125 ppm, which is characteristic of sp2-hybridized carbon in condensed aromatic rings. The line width of the signal is much broader for CW (FWHM = 3120 Hz) than for CChemi (FWHM = 1820 Hz). In principle, the observation of broad resonances can be related to the presence of paramagnetic impurities or a distribution of slightly different chemical environments typical of condensed aromatic carbon systems of different size. EPR spectra (not reported) reveal in both cases the presence of a very small amount of carbon-centred radicals (signals with g-value ∼2.0033). Thus, from the very low density of carbon radicals in both samples, we can surmise that the broader resonance in the 13C CPMAS NMR spectrum of CW is associated with a larger distribution of sp2 islands having a different size. In addition to the signal at 125 ppm, in the spectrum of CChemi a very weak peak is observed around 180 ppm (inset in Fig. 3), which is indicative of the presence of a small amount of oxygen-containing surface functional groups. Although the band is weak and broad, its position is characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups. A similar trend is observed also in the 1H MAS spectra (Fig. S3†). They are characterized by a single broad resonance around 5.5 ppm, with that of CW (FWHM 5600 Hz) broader than that of CChemi (FWHM 2850 Hz).
O functional groups. A similar trend is observed also in the 1H MAS spectra (Fig. S3†). They are characterized by a single broad resonance around 5.5 ppm, with that of CW (FWHM 5600 Hz) broader than that of CChemi (FWHM 2850 Hz).
The two Raman spectra shown in Fig. 4 are both dominated by two intense bands, which are attributed to vibrational modes involving sp2-bonded carbon atoms belonging to disordered microcrystalline domains. In particular, the band centred at 1605 cm−1 (G band) is commonly assigned to the bond stretching of pairs of sp2 carbon atoms (either in aromatic rings or chains),1–3 as schematically shown in the left inset of Fig. 4. For crystalline graphite, the G mode has E2g symmetry and gives a band at about 1580 cm−1. The large shift observed in the present case is common to other disordered graphitic systems and has been explained by considering the overlap of a second band (D2, around 1620 cm−1), which is ascribed to lattice vibrations analogous to that of the G band but involving surface graphene layers, i.e. not sandwiched between two other layers.50–54 Very recently, it has been reported that the C–C stretch Raman fingerprint systematically moves to higher frequency whenever the graphitic molecular nanostructure goes from a planar geometry to a strained geometry.55 On this basis, the position of the G band in the present spectra provides evidence that the graphene stacking order is very low in both carbons, in agreement with XRPD data, and that the graphene layers are at least partially defective.
On the other hand, the origin of the band close to 1350 cm−1 (band D, or D1 in the specialized literature) has been debated for long time; it is usually assigned to a lattice breathing mode with A1g symmetry (right inset of Fig. 4), which is forbidden in ideal graphitic crystals, but becomes Raman active in the presence of structural disorder,1–3,53,54,56 although the theoretical work of Thomsen and Reich ascribes it to a double resonant Raman scattering.57 In particular, it has been suggested to arise from carbon atoms close to the edge of a graphene layer. Hence, the relative ratio between the intensity of the D and G bands – I(D)/I(G) – should correlate with the degree of structural disorder. After the first report of Tuinstra and Koenig,58 who reported that the intensity ratio between the two bands varies inversely with carbon cluster dimension (TK correlation), the I(D)/I(G) value has long been indicative of the size of the graphitic domains in different carbon-based materials. Nevertheless, the extraction of the I(D)/I(G) ratio from the Raman spectra is ambiguous in the literature and large discrepancies are found according to the different evaluation techniques of the Raman spectra. More recently it has been argued that the TK correlation is not applicable for graphitic domains having a lateral dimension smaller than about 2 nm. The argument is based on the fact that the intensity of the D band is proportional to the probability of finding a six-fold ring in the cluster, which is proportional to the cluster area. Therefore, for crystallite sizes below 2 nm the development of a D band in the Raman spectrum indicates ordering, which is exactly the opposite than for graphitic clusters larger than 2 nm.59 It is thus clear that the correlation of the I(D)/I(G) value with structural properties of carbons is not straightforward. In the present case, the Raman spectra of CW and CChemi carbons are clearly characterized by a different I(D)/I(G), the value being higher for CW than for CChemi. According to the XRPD data discussed above, both carbons are characterized by graphitic domains smaller than 2 nm and are more extended for CW than for CChemi. In these conditions the TK correlation is no more valid and the higher I(D)/I(G) value for CW indicates that it is characterized by more ordered sp2 domains with respect to CChemi.
Additional broad absorptions are observed in the Raman spectra of both carbons, around 1450 cm−1 (D3 band, in between the G and the D bands), attributed to a statistical distribution of amorphous carbon on the interstitial position of the graphitic islands60 and around 1150 cm−1 (I band), originating from a coexistence of sp3 and sp2 phases (the last one in the form of conjugated non-aromatic polyenes).61 Both of them have been attributed to stretching vibrations involving the amorphous carbon phase. These bands are relatively more prominent for CChemi, suggesting that the fraction of amorphous carbon is larger than in CW. Finally, in the spectrum of CChemi, a weak but well-resolved band is observed around 1700 cm−1, which indicates the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.62 This observation is in good agreement with the 13C CPMAS SSNMR measurements and confirms that CChemi contains a larger amount of surface oxygen-containing groups.
O groups.62 This observation is in good agreement with the 13C CPMAS SSNMR measurements and confirms that CChemi contains a larger amount of surface oxygen-containing groups.
3.4 Surface properties of CW and CChemi and functional groups
With the aim to investigate in detail the surface properties of the two activated carbons at a molecular level we carried out a thorough investigation by means of three techniques which are sensitive to the surface species: XPS, DRIFT and INS spectroscopy. | ||
| Fig. 5 High-resolution XPS spectra of C 1s peak for CW (part a) and CChemi (part b) and corresponding fits with six Gaussian components. For details see Tables S2 and S3.† | ||
The relative atomic concentration O/C in CChemi is about double that in CW, indicating that the surface of CChemi is more oxidized than that of CW. As a consequence, the main C 1s band (band B) appears narrower in the spectrum of CW than in CChemi. Table S4† compares the relative amount of the different oxygenated species, as obtained by normalizing the area of the six deconvoluted bands in the XPS spectra to the area of peak B, set arbitrarily to 100. In both carbons, the most abundant oxygenated species are C–OH or C–O–C (band C).
The assignment of the main absorption bands is not trivial, also because several discrepancies are present in the literature. However, the following assignments seem to be now firmly established:
i) The narrow and prominent absorption band centred around 1600 cm−1, whose assignment has been controversial for a long time, is assigned to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) vibrational modes of conjugated sp2 bonds belonging to graphitic islands.74 The intensity of this band would be reinforced by the presence of oxygen atoms, most likely because of an increase in the dipole moment associated with these ring vibrations. This band is more intense in the spectrum of CChemi that, according to Raman, XPS and 13C SSNMR data, contains a larger amount of oxygenated species.
C) vibrational modes of conjugated sp2 bonds belonging to graphitic islands.74 The intensity of this band would be reinforced by the presence of oxygen atoms, most likely because of an increase in the dipole moment associated with these ring vibrations. This band is more intense in the spectrum of CChemi that, according to Raman, XPS and 13C SSNMR data, contains a larger amount of oxygenated species.
ii) The intense and very broad absorption in the 1300–1000 cm−1 region is due to the overlap of many absorption bands difficult to be distinguished and due to many vibrational modes. In-plane C–H bending modes should contribute in this region,74 but they are necessarily strongly coupled with the collective modes of the carbonaceous C–C skeleton, whose frequency range increases with the size of the system. This is the case not only for the C–H vibration, but also for any other modes associated to geometrical distortion of the C–C skeleton.75 This is the main reason why no specific absorption bands are observed in this frequency range. In addition, most of the oxygenated species detected by XPS may give absorption in this region. Finally, it is important to note that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of phosphonate groups, which have been detected on the CChemi sample by 31P CPMAS SSNMR technique, should also contribute in this region (1380–1140 cm−1).76 Although it is not possible to exclude that a fraction of the total absorbance in the 1300–1000 cm−1 region for CChemi is due to the presence of surface phosphonates, we are inclined to exclude that this is the main reason for the much higher intensity of the DRIFT spectrum of the CChemi sample.
O stretching vibration of phosphonate groups, which have been detected on the CChemi sample by 31P CPMAS SSNMR technique, should also contribute in this region (1380–1140 cm−1).76 Although it is not possible to exclude that a fraction of the total absorbance in the 1300–1000 cm−1 region for CChemi is due to the presence of surface phosphonates, we are inclined to exclude that this is the main reason for the much higher intensity of the DRIFT spectrum of the CChemi sample.
iii) The series of narrow bands in the 900–750 cm−1 range is characteristic of the IR spectra of many molecules consisting of several condensed rings and are assigned to the out-of-plane vibrations of C–H bonds of condensed ring edges.75 It has been demonstrated that the frequencies of the C–H deformational modes of aromatic species are dependent on the number of adjacent hydrogen atoms. According to the nomenclature proposed by Zander, for substituted benzenes the following values were reported: 860–910 cm−1 for an isolated hydrogen atom (solo), 800–810 cm−1 and 810–860 cm−1 for two adjacent hydrogen atoms (duo), and 750–770 cm−1, 770–800 cm−1 and 800–810 cm−1 for three adjacent hydrogen atoms (trio). In the spectrum of the CChemi sample three well-defined bands are observed at 880 cm−1 (solo), 838–807 cm−1 (duo + trio) and 758 cm−1 (trio), which suggest a large heterogeneity of boundaries. The absorption bands due to C–H out-of-plane vibrations are definitely less intense in the spectrum of CW, as expected because of the larger size of the sp2 domains.
iv) Finally, in the spectrum of CChemi a weak but well-defined absorption band is observed at 1707 cm−1, which is assigned to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibrational mode, in good agreement with 13C CPMAS NMR, Raman and XPS data.
O) vibrational mode, in good agreement with 13C CPMAS NMR, Raman and XPS data.
Summarizing, the DRIFT spectra shown in Fig. 6 demonstrate that the surface properties of CW are mainly dictated by terminal C–H bonds, while in CChemi a detectable amount of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups are clearly identified. The presence of these oxygen-containing surface species (and of others, not easily identified by FT-IR but detected by XPS), as well as the reduced dimension of the sp2 islands (as revealed by the previously discussed techniques), might be the main causes for the much higher intensity of the whole DRIFT spectrum for CChemi than for CW. Indeed, an increase in dipole moment associated with the sp2 ring vibrations is expected in the presence of defect sites, including heteroatoms and island terminations.
O functional groups are clearly identified. The presence of these oxygen-containing surface species (and of others, not easily identified by FT-IR but detected by XPS), as well as the reduced dimension of the sp2 islands (as revealed by the previously discussed techniques), might be the main causes for the much higher intensity of the whole DRIFT spectrum for CChemi than for CW. Indeed, an increase in dipole moment associated with the sp2 ring vibrations is expected in the presence of defect sites, including heteroatoms and island terminations.
All the bands observed in the spectra are due to vibrational modes that involve significant hydrogen displacement, since the neutron cross section for the hydrogen nucleus is one order of magnitude higher than that of all the other elements. The two spectra are very similar, although the total intensity is almost two times higher for CChemi than for CW. This means that the type of hydrogen terminations and their relative abundance are basically the same in the two carbons but more abundant in CChemi than in CW. The larger amount of hydrogen termination in the CChemi sample is only partially explained in terms of the higher surface area, which would account only for a factor of 1.5. Hence, we should conclude that in CChemi carbon the sp2 domains are smaller than in CW, in good agreement with previously discussed results. However, it is worth noting that discrepancies between the intensity of the INS spectra and the hydrogen content in carbon materials have been reported in the past. In particular, Fillaux et al.77–83 suggested that a part of the hydrogen species in carbons behave like free protons and therefore they respond in a different way. A clear explanation of this phenomenon is still not available.
The absorption band centred around 3060 cm−1 is assigned to ν(C–H) vibrational modes of aromatic species. At the other extreme of the spectra, the weak bands in the 700–400 cm−1 region are mainly due to C–C torsion modes of the carbon atoms at the edge of the fragment, which indirectly cause a substantial movement of the hydrogen atoms (riding vibrations).83 It is worth noting that also COOH functional groups may weakly contribute in this vibrational region.83 The broad bands around 1200 cm−1 and in the 800–1000 cm−1 region are due to the in-plane and out-of-plane C–H bending modes of hydrogen species belonging to condensed ring edges.78 In particular, in the latter region the most prominent bands are observed at 952, 880, 830, 800 and 760 cm−1. Most of these bands coincide with those observed in the DRIFT spectra and assigned in terms of solo, duo and trio structures. In particular, according to the literature78 and to our recent calculations,83 the most intense band at 880 cm−1 is associated to CH out-of-plane vibrations of solo species which vibrate in phase; hence, it is indicative of extended sp2 domains having regular borders. Curiously, the intense band at 952 cm−1 is absent in the DRIFT spectra and it was rarely commented in the literature. Very recently we have proposed an assignment in terms of CH out-of-plane mode of duo, trio and quatro species vibrating not in phase;83 thus, this band is associated with irregular borders of sp2 domains. The relative intensity of this band with respect to that at 880 cm−1 is greater for CChemi, indicating that the sp2 domains in CChemi present less extended and more defective borders.
In summary, INS measurements allowed the full characterization of the hydrogen terminations at the sp2 domains. It was found that the hydrogen content is about double in CChemi, which means that the average size of the graphitic plates is smaller in CChemi. Moreover, the distribution of the hydrogen termination is similar but not the same in the two carbons, and in particular CChemi presents less extended and more irregular borders.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching modes involving pairs or rings of sp2 carbons, i.e. collective modes which are strongly related to the structural order of the sp2 domains (G and D bands in Table 3). Hence, Raman spectra convey structural information mainly on the bulk phase of carbon materials, while surface functional groups are rarely observed.
C) stretching modes involving pairs or rings of sp2 carbons, i.e. collective modes which are strongly related to the structural order of the sp2 domains (G and D bands in Table 3). Hence, Raman spectra convey structural information mainly on the bulk phase of carbon materials, while surface functional groups are rarely observed.
| Raman | DRIFT | INS | |||
|---|---|---|---|---|---|
| Bands (cm−1) | Assignment | Bands (cm−1) | Assignment | Bands (cm−1) | Assignment |
| vw = very weak; w = weak; br = broad; s = strong. | |||||
| 1700 (vw) |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
1707 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
||
| 1605 | G band ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of pairs of sp2 C atoms C) of pairs of sp2 C atoms |
1600 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of sp2 C atoms (enhanced by defects) C) of sp2 C atoms (enhanced by defects) |
||
| 1500 (br) | D3 band amorphous phase | ||||
| 1350 (br) | D band ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of sp2 C atoms close to the edge C) of sp2 C atoms close to the edge |
1300–1100 (br) | C–H in-plane bending + collective modes of the C–C skeleton + vibration of oxygenated groups | ||
| 1200 (w) | I band amorphous phase | 1200 | C–H in-plane bending | ||
| 956 (s) | C–H out-of-plane of duo and trio at irregular borders | ||||
| 880 (w) | C–H out-of-plane of solo | 880 (s) | C–H out-of-plane of solo at extended borders | ||
| 838–807 (w) | C–H out-of-plane of duo and trio | 830 | C–H out-of-plane of duo and trio | ||
| 758 (w) | C–H out-of-plane of duo and trio | 760 | C–H out-of-plane of duo and trio | ||
On the contrary, the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) vibrational modes of conjugated double bonds in graphitic sp2 domains are observed in the IR spectrum of a carbon only in the presence of defects (surface terminations, heteroatoms, functional groups, radical carbon species and others), which are responsible for an increase in the dipole moment associated with the ring vibrations. Consequently, IR spectra bring only indirect information on the bulk properties of carbon-based materials, while they contain direct information on the surface species, including both the C–H groups at the periphery of sp2 clusters (usually characterized by weak absorptions) and the functional groups containing heteroatoms such as oxygen (generally giving more intense absorption bands depending on the specific extinction coefficient).
C) vibrational modes of conjugated double bonds in graphitic sp2 domains are observed in the IR spectrum of a carbon only in the presence of defects (surface terminations, heteroatoms, functional groups, radical carbon species and others), which are responsible for an increase in the dipole moment associated with the ring vibrations. Consequently, IR spectra bring only indirect information on the bulk properties of carbon-based materials, while they contain direct information on the surface species, including both the C–H groups at the periphery of sp2 clusters (usually characterized by weak absorptions) and the functional groups containing heteroatoms such as oxygen (generally giving more intense absorption bands depending on the specific extinction coefficient).
In carbons hydrogen content is limited just to the surface (either in the terminal C–H species or in the oxygen-containing functional groups). This makes INS a technique extremely sensitive to surface species. Most of the bands observed in the INS spectrum of CChemi are also found in the DRIFT spectrum, with a few important differences: i) in the INS spectrum the bands are generally narrower and much more resolved; ii) INS spectra are not subjected to selection rules and then show more bands.
Summarizing, among the three vibrational techniques, Raman spectroscopy (excitation λ = 514 nm) is the only one that gives direct information on the bulk properties of carbons, and structural information on the sp2 domains can be derived provided that XRPD data are available. DRIFT spectroscopy is the technique of choice to qualitatively investigate the presence of functional groups that are usually characterized by high extinction coefficients. However, in some cases different IR absorption bands can overlap, making the interpretation of the spectra difficult; in these cases, coupling FT-IR spectroscopy with XPS might be beneficial for understanding. INS spectroscopy (on carefully dehydrated samples) is definitely the best technique to obtain qualitative and quantitative information on the C–H terminations of the sp2 domains, although it is scarcely informative on the presence of other functional groups. It comes out that the synergic coupling of the three techniques is fundamental to extract from vibrational data also information on the structural properties of activated carbons at a molecular scale.
In order to definitely validate our findings, we have treated the CChemi carbon at 750 °C in inert atmosphere and successively measured the Raman, DRIFT and INS spectra, as shown in Fig. S5† (light grey). In all the three cases, the spectra of the sample treated at high temperature decrease in intensity and become very similar to those of the CW carbon discussed previously. From the discussion above, the much larger intensity of the vibrational spectra of CChemi with respect to CW is attributed to the presence of smaller and more defective sp2 domains in CChemi carbon. Hence, the decrease in the overall spectral intensity upon treatment at high temperature provides evidence that CChemi undergoes a graphitization process. Additional discussion can be found in section S5.†
3.5 Vibrational properties of Pd/C catalysts
Raman, DRIFT and INS spectroscopy were applied to investigate the Pd/CW and Pd/CChemi catalysts, with the aim of evaluating whether the catalyst preparation has an influence on the properties of the carbon support. Indeed, during the catalyst synthesis the original support may undergo modifications at the nano- and microscale level,9 although this is often neglected. Preparation of the Pd/C catalysts involves metal deposition in a basic medium, washing, and thermal treatments. In these conditions, activated carbons can release ashes (that can be washed out or can re-precipitate inside the pores),10 may undergo a partial reconstruction involving defective sp2 domains, and a modification of the surface functional groups may also occur. In turn, the modifications of the carbon support during the catalyst preparation may influence its activity towards reactants and products and can affect the availability of the metal particles during the catalytic process.In a previous work we demonstrated that activated carbons are quite inert toward deposition–precipitation of the active metal phase: only very small variations of surface area and pore volume were observed,9 indicating that catalyst preparation does not involve a large reconstruction of the supports. This is confirmed by the similarity of the Raman spectrum of Pd/CW with that of the pristine CW support, as shown in Fig. 8a, although a slight increase of the absorption band around 1500 cm−1 suggests that in Pd/CW the fraction of amorphous carbon is slightly higher than in CW. Also the DRIFT spectrum of Pd/CW is very similar to that of CW (Fig. 8b), excluding that during catalyst preparation new functional groups are formed at the carbon surface. Finally, in the INS spectrum of Pd/CW the bands associated with C–H out-of-plane vibrations are slightly less intense than for bare CW (Fig. 8c), indicating that a minority of the C–H terminations are involved in the deposition of the active phase. In particular, the band at 880 cm−1, indicative of regular borders, is the most affected one. A similar behaviour was observed for Pd/CChemi.
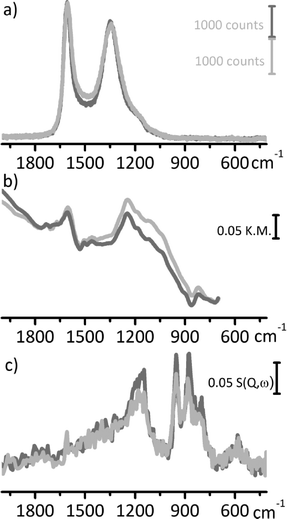 | ||
| Fig. 8 (a) Raman (λ = 514 nm), (b) DRIFT and (c) INS spectra of CW (dark grey) and of the corresponding Pd/CW catalyst (light grey). | ||
The results shown in Fig. 8 demonstrate that the structural and surface properties of Pd/C catalysts are basically the same as those of the pristine carbons. Hence, a comprehensive characterization of the activated carbons with a multi-technique approach as proposed in this work gives all the information useful to tailor the catalyst for a specific purpose.
4. Conclusions
Two couples of Pd/C catalysts, characterized by the same metal dispersion but a different carbon support (CW and CChemi), were tested in two hydrogenation reactions. We found that the catalytic performance strongly depends on the nature of the support. The two carbons were obtained from the same raw material but activated in the presence of either steam or phosphoric acid. Hence, the activation procedure of the carbons has a relevant consequence on the performance of the catalysts. To understand the origin of this effect, we undertook a comprehensive and systematic characterization of the two carbons and of the Pd/C catalysts by a multitude of techniques with different sensitivity and selection rules.We were able to fully understand how the activation procedure influences the morphology, composition, texture, structure and surface properties of the two activated carbons, both at the micro- and at the nanoscale. In particular, it was found that activation in the presence of phosphoric acid leads to the formation of micro-particles smaller than those obtained in the presence of steam. The micro-structure is reflected at a nanoscale: both carbons are mainly graphitic in nature, but CChemi consists of graphitic domains smaller than CW. The sp2 domains have a similar morphology in the two carbons, although in CChemi they are more uniform in size. The surface properties of both activated carbons are determined by terminal C–H bonds and by oxygenated species. The distribution of the hydrogen termination is similar but not the same in the two carbons, and in particular CChemi presents less extended and more irregular borders. CChemi contains more oxygenated species, mainly carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ethers (C–O–C), which contribute to increasing the dipole moment associated with the sp2 ring vibrations and in rendering the whole surface more polar. Finally, CChemi comprises also a detectable amount of phosphorus (at least partially grafted to the carbon in the form of phosphonate species), which is however localized in the bulk and not at the surface, and hence is not relevant for catalysis.
O) and ethers (C–O–C), which contribute to increasing the dipole moment associated with the sp2 ring vibrations and in rendering the whole surface more polar. Finally, CChemi comprises also a detectable amount of phosphorus (at least partially grafted to the carbon in the form of phosphonate species), which is however localized in the bulk and not at the surface, and hence is not relevant for catalysis.
The comprehensive characterization approach proposed in this work allows the rationalization, at least in part, of the role of activated carbons in enhancing the performance of a hydrogenation catalyst. Pd/CW is more selective than Pd/CChemi in the transfer hydrogenation of resorcinol to 1,3-cyclohexanedione. It is a current opinion that during hydrogenation of an aromatic ring over a heterogeneous catalyst (as for the hydrogenation of resorcinol) the hydrogen molecule is split into hydrogen atoms over the metal active phase, while aromatic substrates are adsorbed onto the supports mainly through hydrogen bonds and electrostatic effects.84,85 The adsorbed aromatic ring is then attacked by the adsorbed hydrogen atoms and the hydrogenation reaction occurs. Selectivity is promoted when the product of interest (1,3-cyclohexanedione in our case) is desorbed from the support before undergoing a successive hydrogenation. It is expected that the adsorption of resorcinol onto the carbon support (which is the preliminary step to its hydrogenation) is favoured in the presence of regular sp2 domains. The results discussed in this work demonstrate that CW has larger and more regular sp2 domains than CChemi, and hence correlate the structure of the carbon with the better catalytic performance in the transfer hydrogenation of resorcinol to 1,3-cyclohexanedione. A similar effect was recently reported for a graphene-supported Pd catalyst, which displays excellent selectivity in the same reaction due to the giant π-conjugate interactions between the graphene nano-sheet and the benzene ring of resorcinol.86
On the other hand, the regularity of the sp2 domains in the carbon support is not required for selective catalytic debenzylation reactions, where a high activity for hydrogenolysis should be combined with a low tendency for the reduction of the aromatic rings. In this class of reactions the polarity of the reaction medium is more important, and indeed debenzylation reactions very often are carried out in alcoholic solvents or in acetic acid.22,24 On this basis, it is not surprising that CChemi (which has a more polar and irregular surface, with a larger amount of surface functional groups, as demonstrated in this work) performs better than CW in the debenzylation of ethyl benzyl aniline.
Acknowledgements
We sincerely thank our colleagues and friends Francesca Bonino and Matteo Signorile for the helpful discussion on the Raman spectra, and Ettore Vittone for the discussion on the XPS measurements. C. L. is grateful for support from the Mega-grant of the Russian Federation Government to support scientific research at the Southern Federal University, No. 14.Y26.31.0001.Notes and references
- K.-D. Henning and H. von Kienle, Carbon, 5. Activated Carbon, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2010 Search PubMed.
- R. Schlogl, 2.3.15 Carbons, in Handbook of heterogeneous catalysis, ed. G. Ertl, H. Knozinger, F. Schuth and J. Weitkamp, Wiley-VCH, 2008, vol. 1, p. 357 Search PubMed.
- H. Marsh and F. Rodriguez-Reinoso, in Activated carbon, Elsevier, 2006, p. 13 Search PubMed.
- A. Ahmadpour and D. D. Do, Carbon, 1996, 34, 471 CrossRef CAS.
- B. S. Girgis, S. S. Yunis and A. M. Soliman, Mater. Lett., 2002, 57, 164 CrossRef CAS.
- H. Marsh and P. L. Walker Jr, Fuel Process. Technol., 1979, 2, 61 CrossRef CAS.
- H. Marsh, D. S. Yan, T. M. O'Grady and A. Wennerberg, Carbon, 1984, 22, 603 CrossRef CAS.
- R. Rodrigues, M. Gonçalves, D. Mandelli, P. P. Pescarmona and W. A. Carvalho, Catal. Sci. Technol., 2014, 4, 2293 CAS.
- R. Pellegrini, G. Leofanti, G. Agostini, E. Groppo, M. Rivallan and C. Lamberti, Langmuir, 2009, 25, 6476 CrossRef CAS PubMed.
- E. J. A. X. Van De Sandt, A. Wiersma, M. Makkee, H. Van Bekkum and J. A. Moulijn, Appl. Catal., A, 1998, 173, 161 CrossRef CAS.
- A. Wiersma, E. J. A. X. Van De Sandt, M. A. Den Hollander, H. Van Bekkum, M. Makkee and J. A. Moulijn, J. Catal., 1998, 177, 29 CrossRef CAS.
- L. Faba, E. Díaz and S. Ordóñez, Catal. Sci. Technol., 2015, 5, 1473 CAS.
- M. Galhetas, M. A. Andrade, A. S. Mestre, E. Kangni-Foli, M. J. Villa De Brito, M. L. Pinto, H. Lopes and A. P. Carvalho, Phys. Chem. Chem. Phys., 2015, 17, 12340 RSC.
- H. Teng, T.-S. Yeh and L.-Y. Hsu, Carbon, 1998, 36, 1387 CrossRef CAS.
- J. Laine and S. Yunes, Carbon, 1992, 30, 601 CrossRef CAS.
- G. A. Zickler, B. Smarsly, N. Gierlinger, H. Peterlik and O. Paris, Carbon, 2006, 44, 3239 CrossRef CAS.
- S. Biniak, G. Szymański, J. Siedlewski and A. Świtkowski, Carbon, 1997, 35, 1799 CrossRef CAS.
- J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas and J. J. M. Órfão, Carbon, 1999, 37, 1379 CrossRef CAS.
- http://www.chimet.com/en/catalyst .
- G. Agostini, C. Lamberti, R. Pellegrini, G. Leofanti, F. Giannici, A. Longo and E. Groppo, ACS Catal., 2014, 4, 187 CrossRef CAS.
- R. A. W. Johnstone and A. H. Wilby, Chem. Rev., 1985, 129 CrossRef CAS.
- H.-U. Blaser, A. Indolese, A. Schnyder, H. Steiner and M. Studer, J. Mol. Catal. A: Chem., 2001, 173, 3 CrossRef CAS.
- V. Elango and S. Rajagopal, US Pat., 5744648, 1998 Search PubMed.
- K. G. Griffin, S. Hawker and M. A. Batti, in Catalysis of organic Reactions, ed. R. E. Malz, Marcel Dekker, Inc., New York, 1996, p. 325 Search PubMed.
- R. Pellegrini, G. Agostini, E. Groppo, A. Piovano, G. Leofanti and C. Lamberti, J. Catal., 2011, 280, 150 CrossRef CAS.
- D. Colognesi, M. Celli, F. Cilloco, R. J. Newport, S. F. Parker, V. Rossi-Albertini, F. Sacchetti, J. Tomkinson and M. Zoppi, Appl. Phys. A: Mater. Sci. Process., 2002, 74, S64 CrossRef CAS.
- O. Arnold, J. C. Bilheux, J. M. Borreguero, A. Buts, S. I. Campbell, L. Chapon, M. Doucet, N. Draper, R. Ferraz Leal, M. A. Gigg, V. E. Lynch, A. Markvardsen, D. J. Mikkelson, R. L. Mikkelson, R. Miller, K. Palmen, P. Parker, G. Passos, T. G. Perring, P. F. Peterson, S. Ren, M. A. Reuter, A. T. Savici, J. W. Taylor, R. J. Taylor, R. Tolchenov, W. Zhou and J. Zikovsky, Nucl. Instrum. Methods Phys. Res., Sect. A, 2014, 764, 156 CrossRef CAS.
- J. R. Anderson and K. C. Pratt, in Introduction to Characterization and Testing of Catalysts, Academic Press, Sydney, Australia, 1986, p. 1 Search PubMed.
- G. Agostini, R. Pellegrini, G. Leofanti, L. Bertinetti, S. Bertarione, E. Groppo, A. Zecchina and C. Lamberti, J. Phys. Chem. C, 2009, 113, 10485 CAS.
- G. Prelazzi, M. Cerboni and G. Leofanti, J. Catal., 1999, 181, 73 CrossRef CAS.
- J. J. Venter and M. A. Vannice, Inorg. Chem., 1989, 28, 1634 CrossRef CAS.
- G. Gokagac, J. M. Leger and F. Hahn, Z. Naturforsch., B: J. Chem. Sci., 2003, 58, 423 Search PubMed.
- S. Bertarione, C. Prestipino, E. Groppo, D. Scarano, G. Spoto, A. Zecchina, R. Pellegrini, G. Leofanti and C. Lamberti, Phys. Chem. Chem. Phys., 2006, 8, 3676 RSC.
- H. Benaddi, D. Legras, J. N. Rouzaud and F. Beguin, Carbon, 1998, 36, 306 CrossRef CAS.
- T. Budinova, E. Ekinci, F. Yardim, A. Grimm, E. Björnbom, V. Minkova and M. Goranova, Fuel Process. Technol., 2006, 87, 899 CrossRef CAS.
- A. M. Puziy, O. I. Poddubnaya, A. Martınez-Alonso, F. Suárez-Garcıa and J. M. D. Tascón, Carbon, 2002, 40, 1493 CrossRef CAS.
- A. M. Puziy, O. I. Poddubnaya, R. P. Socha, J. Gurgul and M. Wisniewski, Carbon, 2008, 46, 2113 CrossRef CAS.
- H. Fujimoto, Carbon, 2003, 41, 1585 CrossRef CAS.
- C. R. Houska and B. E. Warren, J. Appl. Phys., 1954, 25, 1503 CrossRef CAS.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686 CrossRef CAS.
- T. D. Shen, W. Q. Ge, K. Y. Wang, M. X. Quan, J. T. Wang, W. D. Wei and C. C. Koch, Nanostruct. Mater., 1996, 7, 393 CrossRef CAS.
- A. Cuesta, P. Dhamelincourt, J. Laureyns, A. Martinez-Alonso and J. M. D. Tascon, J. Mater. Chem., 1998, 8, 2875 RSC.
- C. A. Johnson, J. W. Patrick and K. Mark Thomas, Fuel, 1986, 65, 1284 CrossRef CAS.
- T. Ungár, J. Gubicza, G. Ribárik, C. Pantea and T. W. Zerda, Carbon, 2002, 40, 929 CrossRef.
- R. K. Harris, in Encyclopedia of Magnetic Resonance, ed. R. E. Wasylishen, S. E. Ashbrook and S. Wimperis, John Wiley & Sons Ltd, Chichester, UK, 2012, p. 857 Search PubMed.
- R. K. Harris, R. E. Wasylishen and M. J. Duer, NMR Crystallography, John Wiley & Sons Ltd, Chichester, UK, 2009 Search PubMed.
- M. R. Chierotti and R. Gobetto, CrystEngComm, 2013, 15, 8599 RSC.
- N. Wada, P. J. Gaczi and S. A. Solin, J. Non-Cryst. Solids, 1980, 35–36(Part 1), 543 CrossRef CAS.
- K. W. R. Gilkes, H. S. Sands, D. N. Batchelder, J. Robertson and W. I. Milne, Appl. Phys. Lett., 1997, 70, 1980 CrossRef CAS.
- A. Sadezky, H. Muckenhuber, H. Grothe, R. Niessner and U. Pöschl, Carbon, 2005, 43, 1731 CrossRef CAS.
- Y. Wang, D. C. Alsmeyer and R. L. McCreery, Chem. Mater., 1990, 2, 557 CrossRef CAS.
- O. Beyssac, B. Goffé, J.-P. Petitet, E. Froigneux, M. Moreau and J.-N. Rouzaud, Spectrochim. Acta, Part A, 2003, 59, 2267 CrossRef.
- C. Castiglioni, M. Tommasini and G. Zerbi, Philos. Trans. R. Soc., A, 2004, 362, 2425 CrossRef CAS PubMed.
- M. Tommasini, C. Castiglioni, G. Zerbi, A. Barbon and M. Brustolon, Chem. Phys. Lett., 2011, 516, 220 CrossRef CAS.
- H. A. Galué, Chem. Sci., 2014, 5, 2667 RSC.
- A. C. Ferrari, Solid State Commun., 2007, 143, 47 CrossRef CAS.
- C. Thomsen and S. Reich, Phys. Rev. Lett., 2000, 85, 5214 CrossRef CAS PubMed.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- T. Jawhari, A. Roid and J. Casado, Carbon, 1995, 33, 1561 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 121405 CrossRef.
- D. Lin-Vien, N. B. Colthup, W. G. Fateley and J. G. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Academic Press, London, UK, 1991 Search PubMed.
- NIST X-ray Photoelectron Spectroscopy Database, http://srdata.nist.gov/xps/.
- J. Zawadzki, B. Azambre, O. Heintz, A. Krztoń and J. Weber, Carbon, 2000, 38, 509 CrossRef CAS.
- J. Zawadzki and M. Wiśniewski, Carbon, 2003, 41, 2257 CrossRef CAS.
- D. B. Mawhinney and J. T. Yates Jr, Carbon, 2001, 39, 1167 CrossRef CAS.
- B. J. Meldrum and C. H. Rochester, J. Chem. Soc., Faraday Trans., 1990, 86, 861 RSC.
- B. J. Meldrum and C. H. Rochester, J. Chem. Soc., Faraday Trans., 1990, 86, 1881 RSC.
- B. J. Meldrum and C. H. Rochester, J. Chem. Soc., Faraday Trans., 1990, 86, 2997 RSC.
- B. J. Meldrum and C. H. Rochester, J. Chem. Soc., Faraday Trans., 1990, 86, 3647 RSC.
- C. Moreno-Castilla, F. Carrasco-Marín and A. Mueden, Carbon, 1997, 35, 1619 CrossRef CAS.
- C. Moreno-Castilla, M. A. Ferro-Garcia, J. P. Joly, I. Bautista-Toledo, F. Carrasco-Marín and J. Rivera-Utrilla, Langmuir, 1995, 11, 4386 CrossRef CAS.
- C. Moreno-Castilla, N. V. Lòpez-Ramòn and F. Carrasco-Marín, Carbon, 2000, 38, 1995 CrossRef CAS.
- A. C. Ferrari, S. E. Rodil and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 155306 CrossRef.
- A. Centrone, L. Brambilla, T. Renouard, L. Gherghel, C. Mathis, K. Müllen and G. Zerbi, Carbon, 2005, 43, 1593 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley & Sons, New York, 1978 Search PubMed.
- P. W. Albers, S. Bösing, H. Lansink Rotgerink, D. K. Ross and S. F. Parker, Carbon, 2002, 40, 1549 CrossRef CAS.
- P. W. Albers, J. Pietsch, J. Krauter and S. F. Parker, Phys. Chem. Chem. Phys., 2003, 5, 1941 RSC.
- F. Fillaux, R. Papoular, S. M. Bennington and J. Tomkinson, J. Non-Cryst. Solids, 1995, 188, 161 CrossRef CAS.
- F. Fillaux, R. Papoular, A. Lautié and J. Tomkinson, Carbon, 1994, 32, 1325 CrossRef CAS.
- F. Fillaux, R. Papoular, A. Lautié and J. Tomkinson, Fuel, 1995, 74, 865 CrossRef CAS.
- P. J. R. Honeybone, R. J. Newport, J. K. Walters, W. S. Howells and J. Tomkinson, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 839 CrossRef CAS.
- A. Piovano, A. Lazzarini, R. Pellegrini, G. Leofanti, G. Agostini, S. Rudic, A. L. Bugaev, C. Lamberti and E. Groppo, Adv. Condens. Matter Phys., 2015, 2015, 803267 Search PubMed.
- C. A. Hunter, Chem. Soc. Rev., 1994, 23, 101 RSC.
- C. A. Hunter, K. R. Lawson, J. Perkins and C. J. Urch, J. Chem. Soc., Perkin Trans. 2, 2001, 651 RSC.
- Z. Wei, R. Pan, Y. Hou, Y. Yang and Y. Liu, Sci. Rep., 2015, 5, 15664 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The chemical composition of the two carbons determined by EDX analysis, 1H CPMAS NMR spectra of the two carbons. See DOI: 10.1039/c6cy00159a |
| This journal is © The Royal Society of Chemistry 2016 |






