Graphene decorated with Fe nanoclusters for improving the hydrogen sorption kinetics of MgH2 – experimental and theoretical evidence†
M. Sterlin Leo
Hudson
*a,
Keisuke
Takahashi
 *b,
A.
Ramesh
a,
Seema
Awasthi
c,
Ashish Kumar
Ghosh
d,
Ponniah
Ravindran
a and
Onkar Nath
Srivastava
e
*b,
A.
Ramesh
a,
Seema
Awasthi
c,
Ashish Kumar
Ghosh
d,
Ponniah
Ravindran
a and
Onkar Nath
Srivastava
e
aDepartment of Physics, Central University of Tamil Nadu, Thiruvarur – 610101, India. E-mail: msterlinleo@cutn.ac.in; Tel: +91 9486 860214
bGraduate School of Engineering, Hokkaido University, Sapporo 060-8278, Japan. E-mail: keisuke.takahashi@eng.hokudai.ac.jp; Tel: +81 11 706 6772
cSchool of Physics, Hyderabad Central University, Hyderabad-500046, India
dCentral Institute of Mining and Fuel Research, Dhanbad-826001, India
eDepartment of Physics, Banaras Hindu University, Varanasi-221005, India
First published on 7th August 2015
Abstract
Graphene decorated with Fe clusters is proposed to be a possible alternative catalyst for the hydrogenation and dehydrogenation reactions of MgH2. In particular, graphene decorated with Fe clusters is effective for both hydrogenation and dehydrogenation processes of MgH2. The change in enthalpy and entropy values of hydrogen absorption determined for MgH2 with 5 wt% graphene decorated with Fe clusters is −50.4 ± 2.9 kJ per mole of H2 and 99.8 ± 5.2 J K−1 per mole of H2, respectively. This is significantly lower than those for well-established metal catalysts and nano-interfacial confined MgH2. Moreover, the graphene decorated with Fe clusters facilitates the fast rehydrogenation kinetics of MgH2, which reabsorbed 90% of the total reabsorption capacity in less than 4 minutes at 300 °C and 20 atm. In addition, TEM analysis reveals that MgH2 particles are covered by graphene with Fe clusters, resulting in the reduction of grain growth. Density functional theory shows that the defects in graphene act as the active sites for the dehydrogenation of MgH2, while the Fe clusters reduce the adsorption of dissociated H atoms, resulting in low-temperature dehydrogenation. Thus, graphene decorated with metal clusters could open up a new way of designing a new type of catalyst which could replace transition metal catalysts.
Introduction
Functionalized graphene has quickly caught much attention due to its unexpected properties in the field of catalysis.1 Graphene oxide is a successful modification of graphene, where graphene oxide shows high catalytic activity during oxidation processes.2,3 In the same fashion, metal cluster-decorated graphene happens to have unexpected catalytic effects on various systems.4–6 In particular, graphene decorated with iron clusters is experimentally and theoretically found to store hydrogen.7,8 During the hydrogen absorption process over graphene with iron clusters, calculations also revealed that H2 dissociation occurs over iron clusters, which is considered to cause spillover towards graphene.7 Such a phenomenon can be a key reaction for the hydrogenation process as H2 dissociation is generally the first reaction step of the hydrogenation reaction. Here, magnesium hydride (MgH2) is chosen to evaluate the catalytic effect of graphene with Fe nanoclusters. Magnesium hydride is a potential hydrogen storage material containing 7.6 wt% H2 (theoretical capacity). However, an effective catalyst is required to overcome the high thermodynamic stability and slow hydrogenation reaction kinetics of MgH2. The reaction of magnesium hydride is a prototype hydrogenation reaction which requires Mg, H2 and MgH2 dissociation. In order to improve the kinetics of the hydrogenation and dehydrogenation of magnesium hydride, tremendous amounts of catalysts have been extensively investigated.9–14 However, the search for better catalysts to accelerate the kinetics, hydrogenation, and dehydrogenation of MgH2 is still a problematic issue for further practical applications. Metal-decorated graphene catalysts could be proposed to be potential catalysts for the hydrogenation reaction of such systems. The catalytic effect of graphene decorated with iron clusters is experimentally and theoretically investigated.In particular, MgH2 is used as a prototype reaction in order to evaluate the catalytic effect of graphene decorated with iron towards the hydrogenation and dehydrogenation process. Hydrogen absorption and desorption of MgH2 with graphene decorated with iron clusters are experimentally tested. Transmission electron microscope imaging is used to observe the microstructure of graphene, iron, and MgH2. Density functional theory is implemented in order to reveal the physical and chemical origin of the reaction mechanism. Although the study utilizes iron-decorated graphene and MgH2, the catalytic effect and mechanism of graphene with metal systems should be generalized to further develop metal-decorated graphene catalysts.
Experimental
MgH2 powder of 98% purity and Fe nanopowder (APS 10–30 nm) of 99.9% purity were purchased from Alfa Aesar. Graphene decorated with Fe nanoclusters (G-Fe) was synthesized in an electric arcing chamber using graphite electrodes. To ablate the material, an electric arc was generated using a DC source of 100 A and 25 V between a pure graphite rod (cathode) placed opposite to a grounded anode rod made of graphite impregnated with Fe nanopowder in an arcing chamber filled with Ar (99.999% purity) with a partial pressure of ~300 torr. After 8–10 min of the arcing process, a flake-like carbon material was found to be deposited around the walls of the arcing chamber which was later confirmed as G-Fe. The MgH2 powder was ball-milled together with 5 wt% G-Fe under 5 atm hydrogen pressure in a custom fabricated stainless steel vial of 250 cm3 volume (capable of retaining up to 60 atm pressure) using a Retsch PM 400 planetary ball-miller at an operating speed of 150 rpm for a period of 60 minutes (6 × 10 minutes ball-milling with 15 minute interval in-between). The ball-to-powder ratio was kept at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where the weight of the sample was around 2.5 grams. Ball-milling under hydrogen atmosphere prevents MgO formation.13 For comparative analysis, MgH2 (without G-Fe) was ball-milled under identical experimental conditions for 5 hours (30 × 10 minutes ball-milling with 15 minute interval in-between). Handling of the samples was done in an mBraun MB10 compact Ar-filled glove box (H2O and O2 levels <1 ppm).
1, where the weight of the sample was around 2.5 grams. Ball-milling under hydrogen atmosphere prevents MgO formation.13 For comparative analysis, MgH2 (without G-Fe) was ball-milled under identical experimental conditions for 5 hours (30 × 10 minutes ball-milling with 15 minute interval in-between). Handling of the samples was done in an mBraun MB10 compact Ar-filled glove box (H2O and O2 levels <1 ppm).
The dehydrogenation and reabsorption kinetics of the samples were analyzed through temperature-programmed desorption (TPD) and pressure–composition isotherm (PCI) measurement using an automated four channel volumetric Sievert-type apparatus supplied by Advanced Materials Corporation. TPD analysis of the sample was done at an initial pressure of 10−3 torr under dynamic heating conditions with an accuracy of ±0.2 °C. The rehydrogenation kinetics was measured at different temperatures by charging the dehydrogenated MgH2 samples with 20 atm hydrogen pressure. Thermal analyses of the samples were conducted using a differential scanning calorimeter (DSC 8000, PerkinElmer). The samples were heated from room temperature to about 500 °C with a set heating rate under flowing argon of 20 ml min−1.
Structural characterization of the samples was performed by X-ray diffraction using a PANalytical X'Pert PRO diffractometer with a Cu Kα beam (λ = 1.5415 Å) operated at 40 kV and 40 mA. The samples were loaded in airtight sample holders sealed by a fine layer of parafilm (Pechiney plastic packing) to prevent the sample from being contaminated by oxygen and moisture.14 The microstructures of the samples were analyzed through bright field imaging and selected-area electron diffraction (SAED) using a FEI Tecnai 20G2 transmission electron microscope (TEM) operated at 200 keV.
Results and discussion
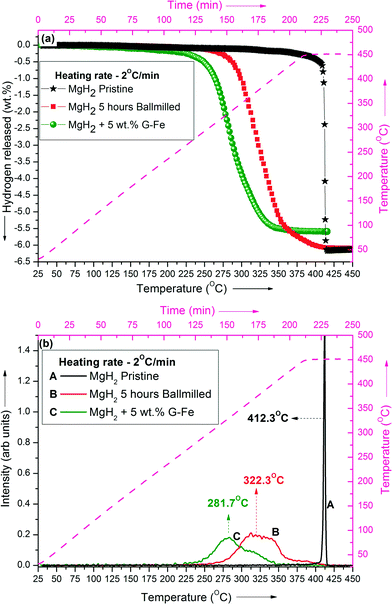
TPD of MgH2 samples was performed from room temperature (25 °C) to 450 °C under dynamic heating conditions at a heating rate of 2 °C min−1. A comparison of the TPD profiles of pristine MgH2, 5 hour ball-milled MgH2 (un-catalyzed) and MgH2 + 5 wt% G-Fe is shown in Fig. 1(a) & (b).It has been observed from the TPD profiles that the dehydrogenation temperature of MgH2 + 5 wt% G-Fe is significantly lower than those of pristine and ball-milled MgH2. The peak temperature of MgH2 + 5 wt% G-Fe is 281.7 °C, whereas that of 5 hour ball-milled MgH2 is 322.3 °C. This suggests that graphene together with Fe nanoclusters exhibits superior catalytic effect in improving the dehydrogenation behaviour of MgH2.
Fig. 2(a) & (b) present the DSC curves of 5 hour ball-milled (un-catalyzed) MgH2 and MgH2 + 5 wt% G-Fe, respectively, determined at different heating rates (2, 5, 10 and 15 °C min−1). The apparent desorption activation energy (Ea) of ball-milled MgH2 and MgH2 + 5 wt% G-Fe was determined using the Kissinger method.15
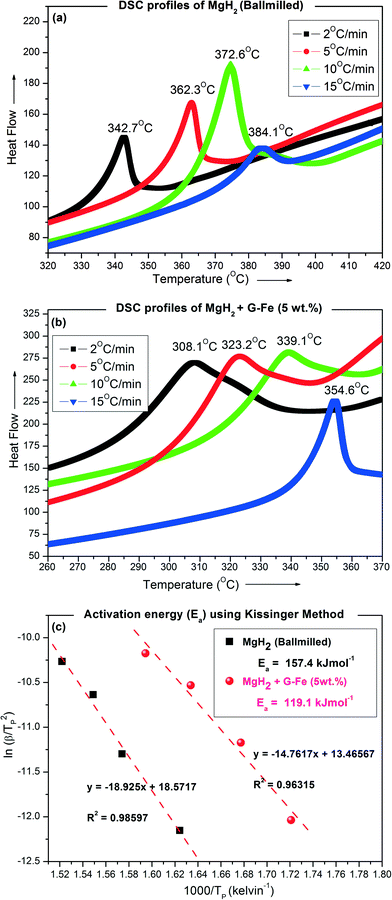 | ||
| Fig. 2 DSC curves measured at different heating rates: (a) ball-milled MgH2 and (b) MgH2 + 5 wt% G-Fe; (c) ln(β/T2P) vs. 1/TP plot. | ||
The peak desorption temperature (TPD) determined from the DSC curves and the corresponding heating rates (β) are the two parameters required for determining Ea using the Kissinger equation given below.
| ln(β/TP2) = (−Ea/RTP) + ln(k0) | (1) |
The desorption activation energy of ball-milled MgH2 (w/o additive) and 5 wt% G-Fe admixed MgH2 determined from the DSC curves using the Kissinger method is 157.4 and 119.1 kJ mol−1. For pristine MgH2, it is ~187 kJ mol−1.16,17Table 1 gives the summary of the calculated apparent desorption activation energy of MgH2 determined from DSC curves using the Kissinger equation.
| Pristine MgH2 | 5 hours ball-milled MgH2 | MgH2 + G-Fe (5 wt%) | |
|---|---|---|---|
| Activation energy (kJ mol−1) | ~187 (ref. 16 and 17) | 157.4 | 119.1 |
It should be noted that 1 hour ball-milled MgH2 + 5 wt% graphene nanosheets (without metal decoration) shows the peak desorption temperature at 358 °C for the DSC heating rate of 5 °C min−1.18 Meanwhile, in the present case, 1 hour ball-milled MgH2 + 5 wt% G-Fe shows the peak desorption temperature at 323 °C for the DSC heating rate of 5 °C min−1 (refer to Fig. 2(b)). Thus the presence of Fe nanoclusters on graphene greatly lowers the dissociation temperature of MgH2.
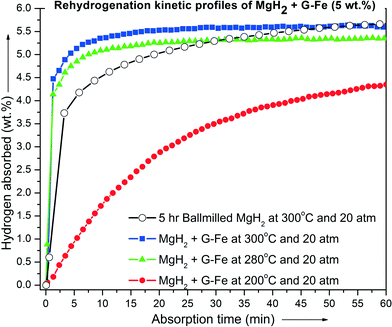
Fig. 3 presents the isothermal reabsorption kinetic curves of dehydrogenated MgH2 + 5 wt% G-Fe determined at different temperatures (200, 280 and 300 °C) and 20 atm hydrogen pressure. It has been observed that the dehydrogenated MgH2 + G-Fe sample shows fast absorption kinetics. The sample absorbed more than 5 wt% hydrogen in less than 4 minutes at 300 °C and 20 atm. Meanwhile, the ball-milled (un-catalyzed) dehydrogenated MgH2 sample took nearly 20 min to absorb 5 wt% hydrogen at 300 °C and 20 atm. This reveals that G-Fe exhibits superior catalytic effect on MgH2 for both hydrogen release and uptake.
Fig. 4(a) & (b) represent the PCI isotherms and Van't Hoff plot of MgH2 + G-Fe, respectively. From the Van't Hoff plot (Fig. 4(b)), the formation enthalpy and entropy values of MgH2 with 5 wt% G-Fe were derived. The change in enthalpy and entropy values is −50.4 ± 2.9 kJ per mole of H2 and 99.8 ± 5.2 J K−1 per mole of H2, respectively, for the formation of MgH2. It should be mentioned that the change in formation enthalpy and entropy values for as-received bulk MgH2 is −74.06 ± 0.42 kJ per mole of H2 and 133.4 ± 0.7 J K−1 per mole of H2, respectively.19 However, the change in formation enthalpy and entropy of MgH2 was reported for element-coated Mg. For Fe-coated Mg, the formation enthalpy and entropy of MgH2 were −59.9 ± 1.9 kJ per mole of H2 and 112.3 ± 3.1 J K−1 per mole of H2, respectively.20
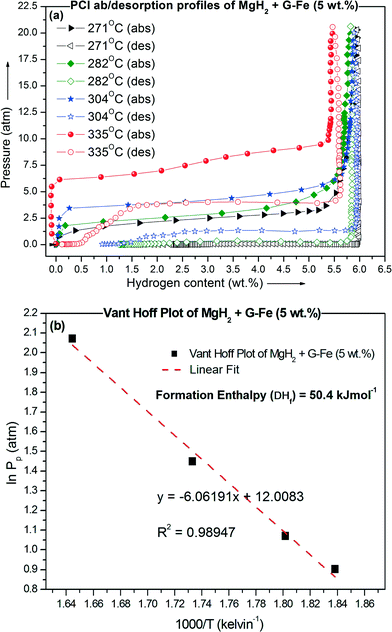 | ||
| Fig. 4 (a) PCI desorption and absorption curves of MgH2 + G-Fe determined at different temperatures and (b) Van't Hoff plot of MgH2 + G-Fe. | ||
Zhao-Karger et al.21 reported that MgH2 nanoparticles (<3 nm) confined in an activated carbon fiber scaffold system exhibit reduced enthalpy and entropy values of −63.8 kJ per mole of H2 and 117.2 J K−1 per mole of H2, respectively. Jia et al.22 observed that MgH2 nanoparticles with ordered mesoporous carbon, CMK-3, exhibit a reduced enthalpy and entropy of −55.4 kJ per mole of H2 and 105.7 J K−1 per mole of H2, respectively. Recently, reduced enthalpy and entropy values of −45 ± 3 kJ per mole of H2 and 84 ± 5 J K−1 per mole of H2, respectively, were observed for MgH2-Ti nanocomposites produced by spark discharge.12
It has been pointed out that the thermodynamic properties of the Mg-to-MgH2 hydrogenation reaction can be dramatically improved at the nanoscale due to the dominant contribution of the high surface to volume ratio.12 In accordance with previous reports, it is known that Fe nanoparticles23 and graphene18,24 are very good catalysts for the MgH2 system. Therefore, in the present investigation, the observation of low reaction enthalpy and entropy values for the hydrogenation reaction of Mg is due to the nanosize and intermixed character of the disordered graphene and Fe nanoparticles. The presence of Fe nanoparticles and graphene is expected to have a synergetic effect in improving the dehydrogenation behavior and in enhancing the hydrogenation of Mg to MgH2 through the spillover effect.25,26
Fig. 5(a) shows the XRD patterns of as-prepared MgH2 + 5 wt% G-Fe, Fig. 5(b) presents the XRD pattern of dehydrogenated MgH2 + G-Fe (5 wt%) after five rehydrogenation cycles and Fig. 5(c) shows its XRD pattern after six rehydrogenation cycles.
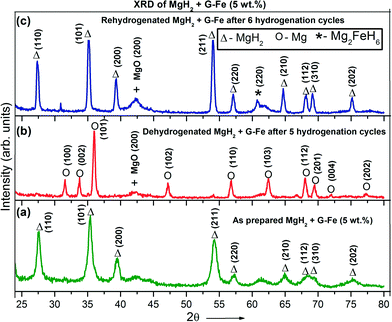 | ||
| Fig. 5 Powder X-ray diffraction patterns of MgH2 + G-Fe: (a) as-prepared, (b) dehydrogenated after five hydrogenation cycles and (c) after six hydrogenation cycles. | ||
It was found that along with the main MgH2 phase, the MgO phase is detected. It was noticed that after six dehydrogenation and rehydrogenation cycles, the diffraction peak intensity increases, suggesting the grain growth of MgH2. Assuming that the MgH2 particles were spherical, the size of the grains were calculated from the XRD peaks using Scherrer's equation.27 This suggests that the average grain size of as-prepared MgH2 + G-Fe (Fig. 5(a)) is 19 nm and after six cycles (Fig. 5(c)), the grain size of MgH2 increases to 34.8 nm. Liu et al.24 observed that after six hydrogenation cycles, the grain size of pure-MgH2 (w/o additives) increased three times (from 10.8 nm before cycling to 32 nm after cycles).
The nucleation and growth of the Mg2FeH6 phase was observed in the cycled MgH2 + G-Fe sample, due to the reaction between elemental Mg and Fe during the hydrogenation process, suggesting that the Fe particles were not free during hydrogenation. However, during dehydrogenation, Mg2FeH6 completely transformed into elemental Mg and Fe, benefiting the initial stage of the rehydrogenation process.28
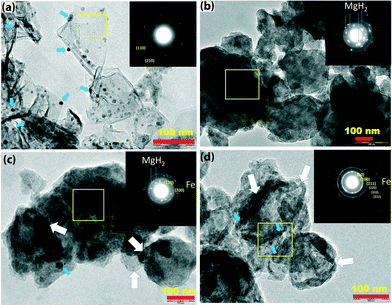
Microstructural analysis of the sample was carried out using TEM. Fig. 6 presents the TEM microstructures of the as-synthesized graphene sheets decorated with Fe nanoclusters (Fig. 6(a)), MgH2 ball-milled for 5 hours (Fig. 6(b)), MgH2 + 5 wt% G-Fe (Fig. 6(c)) and MgH2 + 5 wt% G-Fe after five dehydrogenation/rehydrogenation cycles (Fig. 6(d)).
It was found from the TEM micrograph (Fig. 6(a)) that the Fe nanoparticles (indicated by blue arrows) in G-Fe were distributed throughout the graphene surface. The size of Fe nanoparticles ranges from 5 to 15 nm. The hexagonal pattern observed in the SAED pattern (inset of Fig. 6(a)) confirms that the synthesized sample is graphene. EDAX spectral analysis confirms that the composition of Fe nanoparticles in graphene is 2 wt%. Fig. S1 and S2† show the representative Raman and energy dispersive X-ray (EDX) spectra of as-prepared G-Fe. The distribution of graphene sheets (shown by white arrows) can be readily distinguished in the as-prepared and cycled MgH2 + G-Fe samples (Fig. 6(c) & (d)). The corresponding SAED patterns (insets in Fig. 6(c) & (d)) show the fine grain distribution of the samples. The ring patterns arise in the SAED patterns due to the presence of Fe nanoparticles and the bright patterns are from the MgH2 particles. It has been observed from the TEM micrograph of the cycled sample that graphene is intercalated between MgH2 particles, possibly reducing the agglomeration of MgH2 particles during cycling. In accordance with earlier studies by other researchers, it has been observed that the presence of graphene reduces the grain growth of MgH2, suggesting that graphene is a very good crystal growth inhibitor during dehydrogenation/rehydrogenation cycles of MgH2.25,26
In order to further understand the synergetic effect of Fe nanoclusters and graphene, we have done ab initio studies which are described below.
Computational method
The Grid-based Projector-Augmented Wave method (GPAW) is implemented in density functional theory calculations.29 The grid spacing is set to 0.20 Å with 0.00 eV of smearing. The Γ point is implemented for the sampling of the Brillouin zone. Exchange-correlation of Perdew–Burke–Ernzerhof (PBE) with spin polarization calculations is applied for all calculations.30 Charge transfer analysis is performed using Bader charge analysis.31,32The adsorption energies of the MgH2 cluster and one H atom are calculated using eqn (2)
| Ead = E[Surface+adsorbate] − E[Surface] − E[Adsorbate] | (2) |
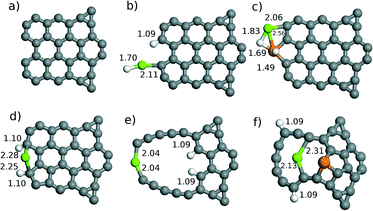
The reaction between Fe-decorated graphene and MgH2 takes place by using the mechanical ball milling process in the experiment. In general, the ball milling process destroys the structure of the material. Hence, atomic clusters are implemented in order to consider the complicated unusual structures. The MgH2 cluster is used in order to understand the fundamental dehydrogenation properties over the Fe-decorated catalysts as MgH2 clusters have been shown to be an effective model to understand the hydrogenation and dehydrogenation properties of MgH2 systems.10 In addition, graphene within non-periodic boundary conditions is considered. In particular, asymmetric graphene consisting of 32 carbon atoms, shown in Fig. 7(a), is constructed within non-periodic boundary conditions.
Computational results
The MgH2 cluster is adsorbed on non-defected graphene with/without an Fe atom, single defected graphene, Fe-doped graphene and large graphene with/without an Fe atom. The adsorption energies of the MgH2 cluster and H atom over each graphene model are collected in Table 2. The structural models are shown in Fig. 7(a–f). The different sizes of defects and the adsorption of Fe monomers are considered based on the graphene in Fig. 7(a).| E MgH2 | E H | |
|---|---|---|
| Non-defected graphene | −4.21 | −0.55 |
| Non-defected graphene + Fe | −1.36 | −0.06 |
| Single defected graphene | −5.38 | −0.83 |
| Large defected graphene | −7.89 | −2.08 |
| Large defected graphene + Fe | −2.48 | −0.53 |
The MgH2 cluster is adsorbed on the edge of non-defected graphene where one of the H atoms is dissociated and adsorbed on the C atom. The adsorption energy of the MgH2 cluster and the H atom on non-defected graphene is calculated to be −4.21 eV and −0.55 eV, respectively. Upon the introduction of Fe atom onto non-defected graphene, both H atoms are fully dissociated from MgH2 clusters, as shown in Fig. 7(c). In particular, it is interesting that the adsorption energy of the MgH2 cluster and H atom dramatically decreased and was −1.36 eV and 0.06 eV compared to the one without Fe. Thus, a dramatic decrease of H adsorption energy is predicted to allow low temperature dehydrogenation. Single C defect is created at the edge of graphene shown in Fig. 7(d). Both H atoms are dissociated over the single C defected graphene. It is interesting that the adsorption energy of the MgH2 cluster on single defected graphene is calculated to be −5.38 eV as single defect enhances the MgH2 cluster adsorption energy by −1.17 eV as compared to the MgH2 cluster on non-defected graphene. Furthermore, H adsorption energy is calculated to be −0.83 eV where H atoms are adsorbed at the edge of graphene shown in Fig. 7(d). However, a decrease of H adsorption energy is not observed in the single defect case compared to the case of Fe-decorated graphene.
A large defect is created in graphene in order to completely break the structure of graphene where six carbon atoms are taken from graphene consisting of 32 C atoms. The structure of graphene is greatly deformed. The adsorption of MgH2 cluster on the large defected graphene particularly shows a high adsorption energy of −7.89 eV where both H atoms are dissociated from the MgH2 cluster, as shown in Fig. 7(e). H adsorption energy is calculated to be −2.08 eV. This also confirms that the edge of the defected graphene is reactive against the MgH2 cluster. However, high H adsorption energy could require higher temperatures for further dehydrogenation processes. An Fe atom is then adsorbed on the large defected graphene shown in Fig. 7(f). The adsorption energy of the MgH2 cluster and H atom over the large defected graphene with Fe is dramatically decreased to −2.84 eV and −0.53 eV, respectively, where full dehydrogenation of the MgH2 cluster is also observed. Thus, one can suggest that the introduction of Fe atom decreases the dehydrogenation temperature of the MgH2 cluster over defected graphene.
Bader charge analysis is performed for the large defected graphene with/without Fe in order to reveal the physical origin of the effect of Fe. Bader analysis indicates that the Fe atom is positively charged by 0.8 electrons. This indicates that electrons from Fe atoms are transferred to C atoms near Fe atoms. One could suggest that Fe atoms fill and stabilize the defects of graphene. As a result, graphene is less active. On the other hand, electrons in the d-orbital of Fe atoms could enhance the dehydrogenation of the MgH2 cluster. Thus, the catalytic activities of defected graphene could be controlled by Fe.
Conclusions
Graphene decorated with Fe clusters is experimentally and theoretically proven to be a possible low cost and alternative catalyst towards the hydrogenation reaction compared to transition metal catalysts. Experiments show that graphene decorated with Fe clusters decreases the dehydrogenation temperature of MgH2 from 322.3 °C to 281.7 °C where the activation energies are experimentally measured to be 119.1 kJ mol−1. Furthermore, the rehydrogenation of MgH2 with graphene decorated with Fe clusters is dramatically improved where it takes only 4 minutes to absorb 5 wt% hydrogen at 300 °C and 20 atm. After six rehydrogenation cycles, the grain size of MgH2 increased only 15 nm, showing low order grain growth during cycling. This is advantageous for MgH2 in maintaining cycling stability, making it suitable for hydrogen storage applications. TEM analysis confirms the low order grain growth of MgH2 and shows that the graphene decorated with Fe clusters behaves like a shell while MgH2 is its core. Density functional theory calculations reveal that the location of the active site – the defect in graphene – and the presence of iron clusters at the defect site of graphene are the key factors for the dehydrogenation of MgH2, as the Fe clusters reduce the adsorption of dissociated H atoms, thus resulting in low-temperature hydrogen release. This suggests that other transition metals could also be effective if charge transfers from the transition metal to graphene are involved. Thus, graphene decorated with Fe clusters could be a potential alternative for transition metal catalysts for hydrogenation reactions and the possibility of utilizing graphene decorated with other metal clusters could further be explored.Acknowledgements
The authors acknowledge the Hydrogen Energy Centre of Banaras Hindu University for providing the necessary experimental facilities. Financial support from the Department of Science and Technology (IFA12-PH-18) and Ministry of New and Renewable Energy (Mission Mode Project) is acknowledged. Thanks are also due to CMPDI, Ministry of Coal, India. One of the authors, Seema Awasthi, acknowledges the University Grants Commission for financial support under the scheme, Dr. DS Kothari PDF grant (no. F.4-2/2006(BSR)/13-768/2012(BSR)). CPU time is funded by the Japan Society for the Promotion of Science. The calculations are performed at Hokkaido University's academic cloud, Information Initiative Center.Notes and references
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications, Chem. Rev., 2012, 112(11), 6156–6214 CrossRef CAS PubMed.
- J. Pyun, Graphene Oxide as Catalyst: Application of Carbon Materials beyond Nanotechnology, Angew. Chem., Int. Ed., 2011, 50(1), 46–48 CrossRef CAS PubMed.
- C. Su, M. Acik, K. Takai, J. Lu, S.-J. Hao, Y. Zheng, P. Wu, Q. Bao, T. Enoki, Y. J. Chabal and K. P. Loh, Probing the catalytic activity of porous graphene oxide and the origin of this behavior, Nat. Commun., 2012, 3, 1298 CrossRef PubMed.
- Y. H. Lu, M. Zhou, C. Zhang and Y. P. Feng, Metal-Embedded Graphene: A Possible Catalyst with High Activity, J. Phys. Chem. C, 2009, 113, 20156–20160 CAS.
- H. R. Byon, J. Suntivich and Y. Shao-Horn, Graphene-based non-noble-metal catalysts for oxygen reduction reaction in acid, Chem. Mater., 2011, 23(15), 3421–3428 CrossRef CAS.
- S. Sabater, J. A. Mata and E. Peris, Catalyst Enhancement and Recyclability by Immobilization of Metal Complexes onto Graphene Surface by Noncovalent Interactions, ACS Catal., 2014, 4(6), 2038–2047 CrossRef CAS.
- K. Takahashi, Y. Wang, S. Chiba, Y. Nakagawa, S. Isobe and S. Ohnuki, Low temperature hydrogenation of iron nanoparticles on graphene, Sci. Rep., 2014, 4, 4598 Search PubMed.
- M. S. L. Hudson, H. Raghubanshi, S. Awasthi, T. Sadhasivam, A. Bhatnager, S. Simizu, S. G. Sankar and O. N. Srivastava, Hydrogen uptake of reduced graphene oxide and graphene sheets decorated with Fe nanoclusters, Int. J. Hydrogen Energy, 2014, 39(16), 8311–8320 CrossRef.
- K. Takahashi, S. Isobe and S. Ohnuki, H2 dissociation over NbO: The first step toward hydrogenation of Mg, Langmuir, 2013, 29(38), 12059–12065 CrossRef CAS PubMed.
- K. Takahashi, S. Isobe and S. Ohnuki, The catalytic effect of Nb, NbO and Nb2O5 with different surface planes on dehydrogenation in MgH2: Density functional theory study, J. Alloys Compd., 2013, 580, S25–S28 CrossRef CAS.
- G. Barkhordarian, T. Klassen and R. Bormann, Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst, Scr. Mater., 2003, 49(3), 213–217 CrossRef CAS.
- A. Anastasopol, T. V. Pfeiffer, J. Middelkoop, U. Lafont, R. J. Canales-Perez, A. Schmidt-Ott, F. M. Mulder and S. W. Eijt, Reduced enthalpy of metal hydride formation for Mg-Ti nanocomposites produced by spark discharge generation, J. Am. Chem. Soc., 2013, 135(21), 7891–7900 CrossRef CAS PubMed.
- T. Sadhasivam, M. S. L. Hudson, S. K. Pandey, A. Bhatnagar, M. K. Singh, K. Gurunathan and O. N. Srivastava, Effects of nano size mischmetal and its oxide on improving the hydrogen sorption behaviour of MgH2, Int. J. Hydrogen Energy, 2013, 38(18), 7353–7362 CrossRef CAS.
- S. K. Pandey, A. Bhatnagar, R. R. Shahi, M. S. L. Hudson, M. K. Singh and O. N. Srivastava, Effect of TiO2 Nanoparticles on the Hydrogen Sorption Characteristics of Magnesium Hydride, J. Nanosci. Nanotechnol., 2013, 13, 5493–5499 CrossRef PubMed.
- H. E. Kissinger, Reaction Kinetics in Differential Thermal Analysis, Anal. Chem., 1957, 29(11), 1702–1706 CrossRef CAS.
- M. Pourabdoli, S. Raygan, H. Abdizadeh and D. Uner, Determination of kinetic parameters and hydrogen desorption characteristics of MgH2-10 wt% (9Ni-2Mg-Y) nano-composite, Int. J. Hydrogen Energy, 2013, 38(27), 11910–11919 CrossRef CAS.
- L. Zhang, L. Chen, X. Xiao, X. Fan, J. Shao, S. Li, H. Ge and Q. Wang, Fluorographene nanosheets enhanced hydrogen absorption and desorption performances of magnesium hydride, Int. J. Hydrogen Energy, 2014, 39(24), 12715–12726 CrossRef CAS.
- G. Liu, Y. Wang, C. Xu, F. Qiu, C. An, L. Li, L. Jiao and H. Yuan, Excellent Catalytic Effects of Highly Crumpled Graphene Nanosheets on Hydrogenation/Dehydrogenation of Magnesium Hydride, Nanoscale, 2013, 5, 1074–1081 RSC.
- M. Paskevicius, D. A. Sheppard and C. E. Buckley, Thermodynamic Changes in Mechanochemically Synthesized Magnesium Hydride Nanoparticles, J. Am. Chem. Soc., 2010, 132(14), 5077–5083 CrossRef CAS PubMed.
- W. Liu, E. J. Setijadi and K. F. Aguey-Zinsou, Tuning the Thermodynamic Properties of MgH2 at the Nanoscale via a Catalyst or Destabilizing Element Coating Strategy, J. Phys. Chem. C, 2014, 118(48), 27781–27792 CAS.
- Z. Zhao-Karger, J. Hu, A. Roth, D. Wang, C. Kübel, W. Lohstroh and M. Fichtner, Altered thermodynamic and kinetic properties of MgH2 infiltrated in microporous scaffold, Chem. Commun., 2010, 46, 8353–8355 RSC.
- Y. Jia, C. H. Sun, N. Cheng, M. A. Wahab, J. Cui, J. Zou and X. Yao, Destabilization of Mg-H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2, Phys. Chem. Chem. Phys., 2013, 15, 5814–5820 RSC.
- M. Ismail, N. Juahir and N. S. Mustafa, Improved Hydrogen Storage Properties of MgH2 Co-Doped with FeCl3 and Carbon Nanotubes, J. Phys. Chem. C, 2014, 118(33), 18878–18883 CAS.
- G. Liu, Y. Wang, L. Jiao and H. Yuan, Understanding the Role of Few-Layer Graphene Nanosheets in Enhancing the Hydrogen Sorption Kinetics of Magnesium Hydride, ACS Appl. Mater. Interfaces, 2014, 6(14), 11038–11046 CAS.
- S. Pevzner, I. Pri-Bar, I. Lutzky, E. Ben-Yehuda, E. Ruse and O. Regev, Carbon Allotropes Accelerate Hydrogenation via Spillover Mechanism, J. Phys. Chem. C, 2014, 118(46), 27164–27169 CAS.
- Y. Lin, F. Ding and B. I. Yakobson, Hydrogen storage by spillover on graphene as a phase nucleation process, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 041402(R) CrossRef.
- A. L. Patterson, The Scherrer Formula for X-Ray Particle Size Determination, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- M. Danaie, A. C. Asselli, J. Huot and G. A. Botton, Formation of the Ternary Complex Hydride Mg2FeH6 from Magnesium Hydride (β-MgH2) and Iron: An Electron Microscopy and Energy-Loss Spectroscopy Study, J. Phys. Chem. C, 2012, 116(49), 25701–25714 CAS.
- J. J. Mortensen, L. B. Hansen and K. W. Jacobsen, Real-space grid implementation of the projector augmented wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 035109 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, Improved grid-based algorithm for Bader charge allocation, J. Comput. Chem., 2007, 28, 899–908 CrossRef CAS PubMed.
- G. Henkelman, A. Arnaldsson and H. A. Jónsson, A fast and robust algorithm for Bader decomposition of charge density, Comput. Mater. Sci., 2006, 36(3), 354–360 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cy01016k |
| This journal is © The Royal Society of Chemistry 2016 |