Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dipolar molecules inside C70: an electric field-driven room-temperature single-molecule switch†
Cina
Foroutan-Nejad
*a,
Valery
Andrushchenko
b and
Michal
Straka
*b
aCEITEC – Central European Institute of Technology, Masaryk University, Kamenice 5/A4, CZ-62500 Brno, Czech Republic. E-mail: cina.foroutannejad@ceitec.muni.cz
bInstitute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám 1, 16610, Prague, Czech Republic. E-mail: straka@uochb.cas.cz
First published on 15th November 2016
Abstract
We propose a two-state electric field-driven room-temperature single-molecule switch based on a dipolar molecule enclosed inside ellipsoidal fullerene C70. We show that the two low-energy minima of the molecular dipole inside the C70 cage provide distinguishable molecular states of the system that can be switched by application of an external electric field.
Current technologies employ about one million atoms for storing a single bit of data.1 Recently, numerous attempts have been made to design and manufacture high-density memory devices which would use just a few atoms to store one bit.2 However, a shortcoming of such devices is that they remain operative only at very low temperatures, e.g., up to 25 K. In this paper we suggest an alternative principle for data storage on the basis of a neutral dipolar molecule encapsulated inside a fullerene. We demonstrate that the dipolar system inside a fullerene can be manipulated by means of an external electric field (EF) that is within the range of electric fields generated by devices, such as a scanning tunneling microscope (STM), which can be as high as 0.5 au (2.57110326 × 1012 V m−1).3 The proposed single-molecule switch may store information on the basis of the orientation of the dipole@fullerene. The hypothesized systems are stable and remain operative under room-temperature conditions.
Endohedral fullerene science has gone through rapid development in the past decade.4 To the best of our knowledge, the idea of manipulating a neutral dipole enclosed in a fullerene has not been pursued before. Several single-molecule switches based on endohedral fullerenes have been proposed.5 Delaney and Greer studied the possibility of manipulating endohedral Li+ by EF inside C60.6 They noted that fullerene behaves as a partial Faraday cage, about 25% of the EF penetrates inside. Yasutake et al. have shown that the Tb@C82 molecule can be oriented on a gold surface by electric field at 13 K.7 Electric current-driven rotation of the endohedral Sc3N cluster in C80 fullerene has been studied at 4.7 K.8 A single-molecule magnetic switch, based on turning on/off the EPR spectra of Sc3C2@C80, has been reported.9 Levitt et al. proposed switches based on ortho- and para-water molecules encapsulated in C60.9,10
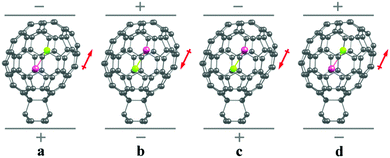
A schematic model of the dipole@fullerene switch proposed here is shown in Fig. 1. The selected fullerene is ellipsoidal, so the internal potential provides two energy minima (states) for the enclosed dipolar molecule, separated by an energy barrier. The barrier should be large enough to prevent the enclosed moiety from spontaneous switching via thermal motion but not too large so that it can be overcome by the interaction of the molecular dipole with an external EF.
An ideal dipolar molecule for building the dipole@fullerene should have a large dipole moment and should be stable under high-temperature conditions that are normal for fullerene synthesis.4b Moreover, the harder (in chemical sense) the endohedral dipole, the higher the stability of the system in high-EF with respect to potential electron transfer from the enclosed molecule to the fullerene cage that is inherently a good electron acceptor.4b
A few fullerenes with neutral dipolar molecule(s) inside their cage have been experimentally prepared so far. These include H2O@C60,11 (H2O)2@C70,12 HF@C60,13 and open-cage fullerenes with CO and H2O enclosed.14 A number of systems have been investigated theoretically, such as LiF, BN, HCN, H2CO, NH3, CO2, and similar molecules enclosed in C60 or C70.15 In a pre-screening study (not discussed here) we investigated various endohedral systems, such as LiF@C70, CO@C70, CH3F@C70, CH3CN@C70, and CH2O@C70.15a,b The energy barrier for turning these molecules inside the C70 cage without an EF was found to be either too low (<3 kcal mol−1) or too large (>50 kcal mol−1 for CH3CN@C70). The barrier was found to be proportional to the size of the molecule. Here we focus on MX@C70-based systems (MX = LiCl, NaF, NaCl), which showed the best properties among the tested systems, having the right size and large dipole moments (>7.0 D). We note that all alkali metal halides are known to exist in the form of diatomic molecules in the gas-phase at high temperatures necessary for the preparation of fullerenes.16 Furthermore, encapsulation of MX molecules in C70 is an exothermic process, see Table S1 (ESI†). Another potentially possible way to prepare such molecules is via chemical opening and closing of a fullerene cage.14
Building a real device ultimately requires that the fullerene is attached to a surface.17 Because linkers including N-heterocyclic carbenes18 and disulfides19 on a gold surface are prone to dissociation under high-EF conditions (due to their bond polarity), we propose to use a nonpolar C–C linker. A hypothetical target system is a graphene sheet, decorated with MX@C70 systems via the Diels–Alder reaction.20
A system in which C70 is attached to a hexane ring21 serves as the smallest model of the fullerene bonded to a graphene sheet, see Fig. 2. We limited our study to the thermodynamically most-stable Diels–Alder product.21 It is worth noting that the EF can catalyze the Diels–Alder reaction20,22 and potentially control the regioselectivity of the reaction between MX@C70 and the graphene surface.23 The reactivity of MX@C70 in the presence of an EF is the subject of our ongoing research to be published subsequently.
Our computations‡ show that the MX aligns nearly along the main axis of symmetry of C70 to minimize the steric clash with the belt region, as expected. Without an EF there are two local minima (LMs), as illustrated in Fig. 1 and 2. With the electric field on, the conformations with the dipole moment of MX along the EF (c and d in Fig. 2) are stabilized more than the other two (a and b in Fig. 2). With increasing EF the stabilization should ultimately lead to the removal of the energy barrier and to the reorientation of the dipole molecule, from a to c (or b to d) in Fig. 2.
The energy dependence of local minima and their interconnecting transition state(s) (TS) on the EF is presented in Fig. 3 (numerical values are given in Table S2, ESI†). Without the EF, the two minima differ slightly in their energy which is due to the presence of the linker. By increasing the EF the energy of the minimum with the dipole aligned parallel to the EF decreases faster as compared to the minimum with the dipole aligned antiparallel to the EF, Fig. 3 and Table S2 (ESI†).
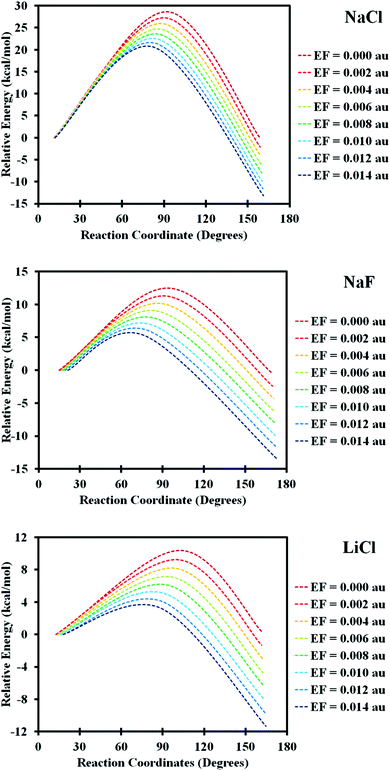 | ||
| Fig. 3 The effect of EF on the energy barrier for rotation of the MX@C70 memory system. (top) NaCl: TSbd, (middle) NaF: TSac, and (bottom) LiCl: TSac. The reaction coordinate is defined by measuring the angle that MX makes with the substituted α-carbon atom (on the pole region of the cage) in the fullerene structure, see Fig. 2. | ||
The energy and structure of the high-energy LM become closer to that of the corresponding TS upon increasing the EF, as expected from classical Hammond's postulate.24 This is the case for all studied systems, see Fig. 3 and Table S2 (ESI†). When extrapolating the results towards a larger EF the internal rotation barrier for MX should disappear, see below. As a result, only one LM should remain on the potential energy surface of the system.25 Note that TSac responds to EF differently from TSbd, Table S2 (ESI†). This is because in the a arrangement the dipole moment of the MX is mostly parallel to the dipole moment of the substituted fullerene cage; therefore, conformer a is more influenced by the EF and reaches the TS more easily.
The changes in the relative energies of the LMs and the TSs are regular in all cases; see Fig. 3 and Table S2 (ESI†). For NaF the barrier is about 13 kcal mol−1 without an EF and goes down to 5(10) kcal mol−1 at EF = 0.014 au for TSac(TSbd). Increasing the size of the internal dipole by replacing NaF with NaCl does not affect the relative energies of the conformers but increases the TS energy by about 100% due to the stronger steric clash between the larger NaCl molecule and the belt region of C70. For a smaller LiF molecule only a small barrier of ∼2 kcal mol−1 was predicted at zero field. Such a barrier can be easily overcome by an EF; for this reason, optimizations of a and b conformers ended up in c and d conformers for EF ≥ 0.004 au and no proper transition states could be located at a higher EF. LiCl “fits” between the largest (NaCl) and the smallest (LiF) systems. Its zero-field barrier is ∼10 kcal mol−1, which prevents the thermal motion switching but the system responds well to the applied EF, see Table S2 (ESI†) and Fig. 3.
Because the response to EF could not be computed beyond 0.014 au as the DFT approximation cannot be trusted after that,‡ we extrapolated the data from Table S2 (ESI†) using the quadratic formula in Fig. 4. While the data for NaF and NaCl diverge, the extrapolated results for LiCl pass the barrier near EF = 0.02 au. The LiCl@C70 system thus appears among studied systems to be the best candidate for the suggested single-molecule switch.
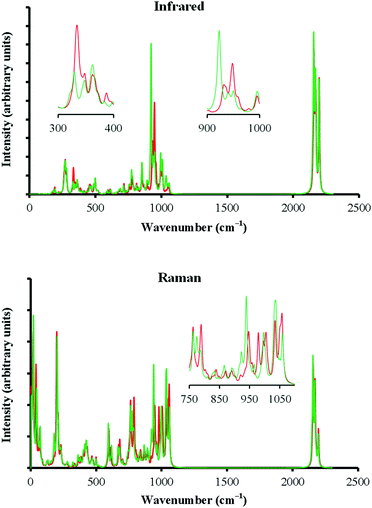
The information can thus be encoded in the MX@C70 systems; but are the two states distinct enough to allow for “the reading” of the device? They are. The two minima of MX@C70 systems differ significantly in their electric multipole moments (Table S3, ESI†) and thus should be distinguishable on the basis of their molecular properties either via STM or spectroscopic methods. Fig. 5 illustrates the differences between IR and Raman spectra of the two minima for LiCl@C70 without an EF (see Fig. S3 and S4 for NaF@C70 and NaCl@C70 spectra, ESI†). While most of the spectral features of both conformers are similar, the differences near 330 and 950 cm−1 in IR spectra and near 800 and 1050 cm−1 in Raman spectra are large enough to be observable experimentally. The detection of a single molecule is still a complicated task but novel advances in single-molecule spectroscopy offer a potential method for reading the dipole@fullerene-type memory devices in the future.26
In summary, we have presented a single-molecule switch that can store information in terms of different orientations of a dipolar molecule inside an ellipsoidal fullerene using model systems of alkali metal halides enclosed in a C70 cage further substituted with cyclohexane to model a carbon-based linker to a graphene sheet. MX molecules have two local energy minima inside the C70 cage separated by an energy barrier due to the shape of the fullerene. The external electric field decreases the energy barrier and enables switching between the states (conformers) of the switch. Extrapolation data suggest that the barrier removal can be achieved at a field of about 0.02 au for LiCl. The calculated IR and Raman spectra of the local minima show that they have distinct molecular properties; hence, reading of the switch is in principle possible.
The project was supported by the Czech Science Foundation (14-03564S) and Czech Academy of Sciences (RVO-61388963). C. F.-N. thanks the SoMoPro II programme, co-financed by the European Union and the South-Moravian Region, REA Grant Agreement No. 291782. This publication reflects only the author's views and the Union is not liable for any use that may be made of the information contained herein. Computational resources were provided by the CESNET LM2015042 and the CERIT Scientific Cloud LM2015085, provided under the program “Projects of Large Research, Development, and Innovations Infrastructures”.
Notes and references
- IBM technology reports, http://https://www-03.ibm.com/press/us/en/pressrelease/36473.wss.
- S. Loth, S. Baumann, C. P. Lutz, D. M. Eigler and A. J. Heinrich, Science, 2012, 335, 196–199 CrossRef CAS PubMed.
- H. Ness, A. J. Fisher and G. A. D. Briggs, Surf. Sci., 1997, 380, L479–L484 CrossRef CAS.
- (a) H. Cong, B. Yu, T. Akasaka and X. Lu, Coord. Chem. Rev., 2013, 257, 2880–2898 CrossRef CAS; (b) A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed; (c) J. Zhao, X. Huang, P. Jin and Z. Chen, Coord. Chem. Rev., 2015, 289, 315–340 CrossRef; (d) Y. Wang, S. Diaz-Tendero, M. Alcami and F. Martin, Nat. Chem., 2015, 7, 927–934 CrossRef CAS PubMed; (e) P. Jin, C. Tang and Z. Chen, Coord. Chem. Rev., 2014, 270, 89–111 CrossRef.
- J. L. Zhang, J. Q. Zhong, J. D. Lin, W. P. Hu, K. Wu, G. Q. Xu, A. T. S. Wee and W. Chen, Chem. Soc. Rev., 2015, 44, 2998–3022 RSC.
- P. Delaney and J. C. Greer, Appl. Phys. Lett., 2004, 84, 431–433 CrossRef CAS.
- Y. Yasutake, Z. J. Shi, T. Okazaki, H. Shinohara and Y. Majima, Nano Lett., 2005, 5, 1057–1060 CrossRef CAS PubMed.
- T. Huang, J. Zhao, M. Feng, A. A. Popov, S. Yang, L. Dunsch and H. Petek, Nano Lett., 2011, 11, 5327–5332 CrossRef CAS PubMed.
- B. Wu, T. Wang, Y. Feng, Z. Zhang, L. Jiang and C. Wang, Nat. Commun., 2015, 6, 6468 CrossRef CAS PubMed.
- (a) S. Mamone, M. Concistrè, E. Carignani, B. Meier, A. Krachmalnicoff, O. G. Johannessen, X. Lei, Y. Li, M. Denning and M. Carravetta, et al. , J. Chem. Phys., 2014, 140, 194306 CrossRef PubMed; (b) B. Meier, S. Mamone, M. Concistrè, J. Alonso-Valdesueiro, A. Krachmalnicoff, R. J. Whitby and M. H. Levitt, Nat. Commun., 2015, 6, 8112 CrossRef CAS PubMed.
- A. Krachmalnicoff, M. H. Levitt and R. J. Whitby, Chem. Commun., 2014, 50, 13037–13040 RSC.
- R. Zhang, M. Murata, T. Aharen, A. Wakamiya, T. Shimoaka, T. Hasegawa and Y. Murata, Nat. Chem., 2016, 8, 435–441 CrossRef CAS PubMed.
- A. Krachmalnicoff, R. Bounds, S. Mamone, S. Alom, M. Concistrè, B. Meier, K. Kouřil, M. E. Light, M. R. Johnson and S. Rols, et al. , Nat. Chem., 2016, 8, 953–957 CrossRef CAS PubMed.
- M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2008, 6083–6094 RSC.
- (a) G. A. Dolgonos and G. H. Peslherbe, Phys. Chem. Chem. Phys., 2014, 16, 26294–26305 RSC; (b) T. Korona and H. Dodziuk, J. Chem. Theory Comput., 2011, 7, 1476–1483 CrossRef CAS PubMed; (c) A. Bil, Z. Latajka and C. A. Morrison, Chem. Phys., 2014, 428, 121–126 CrossRef CAS.
- (a) A. Haaland and M. Tilset, Lewis and Kossel's Legacy: Structure and Bonding in Main-Group Compounds, in The Chemical Bond II: 100 Years Old and Getting Stronger, ed. D. M. P. Mingos, Springer International Publishing, 2016, pp. 1–70 Search PubMed; (b) K. Huber and G. Herzber, Molecular spectra and molecular structure. Constants of diatomic molecules, Van Nostrand, New York, 1979, vol 4 Search PubMed.
- (a) A. L. Balch and M. M. Olmstead, Chem. Rev., 1998, 98, 2123–2166 CrossRef CAS PubMed; (b) S. Osuna, M. Swart and M. Sola, Phys. Chem. Chem. Phys., 2011, 13, 3585–3603 RSC.
- C. M. Crudden, J. H. Horton, I. I. Ebralidze, O. V. Zenkina, A. B. McLean, B. Drevniok, Z. She, H.-B. Kraatz, N. J. Mosey and T. Seki, et al. , Nat. Chem., 2014, 6, 409–414 CrossRef CAS PubMed.
- (a) M. D. Westmeyer, C. P. Galloway and T. B. Rauchfuss, Inorg. Chem., 1994, 33, 4615–4616 CrossRef CAS; (b) J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS PubMed; (c) C. Vericat, M. E. Vela, G. Corthey, E. Pensa, E. Cortes, M. H. Fonticelli, F. Ibanez, G. E. Benitez, P. Carro and R. C. Salvarezza, RSC Adv., 2014, 4, 27730–27754 RSC.
- R. Meir, H. Chen, W. Lai and S. Shaik, ChemPhysChem, 2010, 11, 301–310 CrossRef CAS PubMed.
- J. Mestres, M. Duran and M. Solà, J. Phys. Chem., 1996, 100, 7449–7454 CrossRef CAS.
- A. C. Aragonès, N. L. Haworth, N. Darwish, S. Ciampi, N. J. Bloomfield, G. G. Wallace, I. Diez-Perez and M. L. Coote, Nature, 2016, 531, 88–91 CrossRef PubMed.
- S. Shaik, S. P. de Visser and D. Kumar, J. Am. Chem. Soc., 2004, 126, 11746–11749 CrossRef CAS PubMed.
- G. S. Hammond, J. Am. Chem. Soc., 1955, 77, 334–338 CrossRef CAS.
- (a) S. Grimme, H. Kruse, L. Goerigk and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 1402–1405 CrossRef CAS PubMed; (b) B. Schirmer and S. Grimme, Chem. Commun., 2010, 46, 7942–7944 RSC.
- (a) S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed; (b) P. Roelli, C. Galland, N. Piro and T. J. Kippenberg, Nat. Nanotechnol., 2016, 11, 164–169 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Wallingford CT, 2009 Search PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- C. Foroutan-Nejad, M. Novák and R. Marek, J. Phys. Chem. C, 2015, 119, 5752–5754 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp06986j |
| ‡ Methods: The potential energy hypersurfaces for rotation of the enclosed molecule were investigated by the Gaussian 09 program.27 The local minima and the corresponding transition states to pass from one minimum to another were calculated at different strengths of the electric field using the dispersion-corrected B97D3 functional28 and 6-31+G(d) basis set as implemented in the Gaussian 09 code.27 The fields up to a value of 0.014 au (1.028441304 × 1010 V m−1) were examined in 0.002 au intervals. In fields stronger than the aforementioned field the HOMO–LUMO gap decreases dramatically as a result of state-mixing and DFT computations with contemporary functionals being no longer valid.29 No charge transfer between the carbonic framework and the encapsulated MX was observed in the studied range of the electric field. An EF influences the energy level of a polar molecule the most when it is applied parallel to the largest component of the dipole moment vector. In our study the direction of the EF was selected to be perpendicular to the hypothetical surface that the fullerene is attached to. The transition states and local minima were confirmed by frequency analysis. |
| This journal is © the Owner Societies 2016 |