Energy transduction and signal averaging of fluctuating electric fields by a single protein ion channel
C.
Verdia-Baguena
a,
V.
Gomez
b,
J.
Cervera
c,
P.
Ramirez
*b and
S.
Mafe
c
aDept. de Física, Universitat Jaume I de Castelló, E-12080 Castelló, Spain
bDepartament de Física Aplicada, Universitat Politècnica de València, E-46022 València, Spain. E-mail: patraho@fis.upv.es
cDept. de Física de la Terra i Termodinàmica, Universitat de València, E-46100 Burjassot, Spain
First published on 22nd November 2016
Abstract
We demonstrate the electrical rectification and signal averaging of fluctuating signals using a biological nanostructure in aqueous solution: a single protein ion channel inserted in the lipid bilayer characteristic of cell membranes. The conversion of oscillating, zero time-average potentials into directional currents permits charging of a load capacitor to significant steady-state voltages within a few minutes in the case of the outer membrane porin F (OmpF) protein, a bacterial channel of Escherichia coli. The experiments and simulations show signal averaging effects at a more fundamental level than the traditional cell and tissue scales, which are characterized by ensembles of many ion channels operating simultaneously. The results also suggest signal transduction schemes with bio-electronic interfaces and ionic circuits where soft matter nanodiodes can be coupled to conventional electronic elements.
1. Introduction
Directional fluxes of energy and information occur in a highly fluctuating environment characteristic of biological nanostructures. This is the case of ion channels in cell membranes, the protein aqueous pores which allow the transmission and processing of noisy electrical signals.1 The coexistence of net currents and noise is also significant for developing applications at the nanoscale where fluctuations are usually regarded as a nuisance, not as an opportunity. While the correlation between fluctuations and the state of a system cannot give net currents from equilibrium random fluctuations, this is not the case of non-equilibrium external fluctuations which are uncorrelated with the system state.2It is of wide interest to demonstrate experimental methods allowing the conversion of randomly fluctuating external signals into directional average responses using nanostructures. Previous studies by Siwy3 and us2 have considered the conversion of zero time-average electrical signals into net currents using artificial nanopores. With respect to our previous work,2 the following significant differences should be emphasized: (i) we study here a biological nanoscale system: the outer membrane porin F (OmpF) protein, a bacterial ion channel of Escherichia coli, instead of an artificial polymeric conical nanopore; (ii) the single protein is inserted in the lipid bilayer characteristic of cell membranes1,4 and we use different experimental techniques and equipment because ion channel net currents (pA) are much lower than nanopore currents (nA); (iii) the dimensions of the OmpF protein are of the order of a few nm in radius and length while those of the synthetic conical nanopores used in previous experiments are of the order of ten nm (pore tip), hundreds of nm (pore base) and ten μm (length); (iv) we demonstrate that ion channels can be coupled efficiently with conventional circuit elements, yielding bio-electronic schemes that work in a reproducible and predictable way, which could be exploited in the design of signal averaging and energy transduction schemes using nanopore-based ionic circuits;5–13 (v) the synthetic nanopores used in our previous experiments2 operate in the range of 1–3 V, while the OmpF protein used here shows similar current rectifications in the range of 100–150 mV, thus decreasing the noise voltages needed to charge the capacitor in one order of magnitude; and, finally, (vi) we show that low frequency zero time-average electromagnetic fields could produce noticeable accumulative effects in single ion channels.
Note also that single protein experiments could permit quantification of the signal averaging of fluctuating fields at a more fundamental level than the traditional cell and tissue scales,14 which involve ensembles of many ion channels operating simultaneously. Ion channel selectivity and electric field-sensing are crucial to bioelectrical activity.15,16 Oscillating electrical signals are also observed along the cell cycle17 and in intercellular communication.18–20 The bioelectrical characteristics of commercially available protein ion channels and toxin pore-formers can be used in bio-electronic interfaces and ionic nanodevices;4–7,17 in particular, asymmetric channels allow different electrical rectification phenomena.4,21–23
2. Experimental methods
2.1 Materials
The single OmpF porin reconstituted on a planar diphytanoylphosphatidylcholine (DPhPC) lipid bilayer (Avanti Polar Lipids, Alabaster, AL) separates two 0.1 M KCl aqueous solutions. The reconstitution procedure can be found elsewhere.22 When pHL = 3 and pHR = 12, where L denotes the side of protein addition, the protein channel acts as a biological nanofluidic diode because of the positive (basic) residues on the left side and the negative (acidic) residues on the right side.4 The resulting fixed charge asymmetry along the protein channel leads to the observed rectifying characteristics in the current (I)–voltage (V) curve. The symmetrical case pHL = pHR = 6 gives a quasi-ohmic behavior.2.2 Electrical measurements
The case V > 0 corresponds to the electric potential being higher on the L side than on the R side of the membrane cell. The current I > 0 corresponds to an electric flow from the L solution to the R solution. Voltage and current are measured with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Ag|AgCl electrodes are used in the L and R solutions to externally apply the fluctuating potentials. A WW5062 Tabor Electronics wave generator was used to introduce random noise signals (25 MHz bandwidth). The voltage across the load capacitor is measured with an electrometer (Keithley 6514, input impedance > 200 TΩ, sampling frequency 50 kHz). The electrochemical cell with the lipid bilayer is confined within a double layer magnetic shield (Amuneal Manufacturing, Philadelphia, PA) to avoid external perturbations. The system formed by the single channel inserted in the lipid bilayer has an effective capacitance of the order of 100 pF. Occasionally, two channels instead of one can be inserted in the lipid bilayer, which can be readily detected by the anomalies observed in the time traces for VC(t) during the charging process. In this case, the capacitor charging is still possible but the time constants obtained are not characteristic of the single protein case we wish to study here.3. Results and discussion
Fig. 1a schematically shows the single ion channel protein reconstituted on a planar lipid bilayer which separates two aqueous KCl solutions of concentrations cL = cR = 0.1 M. The electrical charge of the basic and acidic protein residues distributed along the channel axis depends on their ionization state. The preference of the channel for cations or anions (ionic selectivity) is dictated by the solution pH:21,22 the positively charged channel is selective to anions at low pH, whereas the negatively charged channel is selective to cations at high pH. Not only is the ion selectivity but also the I–V curves in Fig. 1b are dictated by the pH values in the external solutions.4 The asymmetric configuration case (pHL ≠ pHR) gives nonlinear I–V curves (rectifying behaviour, with a high conducting regime for V > 0 and a low conducting regime for V < 0), while the symmetrical case (pHL = pHR) gives quasi-linear I–V curves (Fig. 1c and d). In the asymmetric case, the Nernst voltage offset is negligible because of the relatively high concentration of KCl (0.1 M) compared with the water ion concentration.1The channel ionic current is dictated by the potassium and chloride ions in the pore aqueous solution because these ions have concentrations much higher than those of hydrogen and hydroxyl ions under the conditions given in Fig. 1a and c. The water ions allow the biological nanostructure to show a bipolar distribution of fixed charges (Fig. 1a) but do not add a significant contribution to the total current.4,22 The asymmetric charge distribution along the protein pore in Fig. 1a gives the rectification characteristics of the nanofluidic diode in Fig. 1b. The rectification ratio, defined as the ratio of the high conducting regime to the low conducting regime currents and measured at the same absolute value of the voltage, describes the performance of the diode as a rectifier. In the case of the asymmetric configuration of Fig. 1b, the rectification ratio is about 7 at 150 mV. In the synthetic nanopores used in previous experiments,2 applied voltages in the range 1–3 V were needed to obtain similar rectification ratios.
Fig. 2 (top) shows the experimental set-up for the charging process. The electrochemical cell that contains the planar lipid bilayer and the load capacitor of capacitance C connected in series to the protein channel is electromagnetically shielded from external noises. Fig. 2 (left) shows the randomly fluctuating electric potential Vin(t) of zero time-average (white noise) applied externally. The oscillating input signal is characterized by the voltage amplitude V0 and the pulse 0.01 s. The pore rectification shown in Fig. 1b gives the output electric current Iout(t) in Fig. 2 (bottom). The instantaneous current Iout(t) shows no time delay with respect to Vin(t) because the input potential period is much longer than the typical relaxation time of the system4,23 (note the small volume available for ionic conduction within the protein pore of Fig. 1a).
A capacitor voltage VC(t) is set up in Fig. 2 (right) due to the non-zero average current obtained with the oscillating signal in Fig. 2 (left). Because this voltage drives a reverse current which opposes the charging process, the total current decreases to zero when the capacitor approaches the final voltage VC(∞) for large times t → ∞.
Fig. 3a shows the typical time traces measured for the capacitor voltage VC(t) during the charging process for capacitance C = 100 nF. The curves are parametric in the amplitude V0 of the input fluctuating potential. For potential amplitudes in the range of 50–150 mV, the single protein rectification of an external oscillating signal gives significant steady-state capacitor voltages within a few minutes.
Fig. 3b shows the discharging process obtained for the amplitude V0 = 150 mV in Fig. 3a when the external potential is switched off. Fig. 1a suggests a pore resistance of the order of 1 GΩ. For a load capacitance C = 100 nF, the characteristic time of the electrical circuit should then be of the order of 109 Ω × 10−7 F = 100 s, which is in agreement with the results of Fig. 3a and b. Note that the rectification in Fig. 1b can lead to different single channel resistances for the charging and discharging processes.
 | ||
| Fig. 3 (a) Time traces for the capacitor voltage VC(t) during the charging process with a capacitance C = 100 nF in Fig. 2. The curves are parametric in the amplitude V0 of the fluctuating potential. (b) The discharging process for VC(t) in the case V0 = 150 mV in (a). (c) Time traces for VC(t) during the charging process parametric in the capacitances C = 100 nF, 10 nF and 1 nF. (d) The control experiments correspond to the absence of external signals (Vin(t) = 0) and to the symmetric pH case in Fig. 1d for V0 = 100 mV. | ||
To check further that the capacitor charging process is due to the single protein rectification, Fig. 3c considers a broad range of capacitances, C = 1–100 nF. As expected the charging time increases with capacitance. However, high enough capacitance values are required to characterize accurately the capacitor voltage, as shown in Fig. 3c. Indeed, a balance should be reached between the charging time and the capacitor voltage determination. Remarkably, significant accumulative effects resulting in a high value of VC can be obtained within a few seconds for the low capacitance case.
Fig. 3d shows two control experiments corresponding to the absence of an externally applied signal (Vin(t) = 0) and to the symmetric pH case of Fig. 1d. The bottom curve in Fig. 3d shows that the capacitor voltage is close to 1 mV in the absence of external oscillating signals, which is a value significantly lower than most of the observed potentials in Fig. 3a. This result demonstrates that the charging process results from the rectification of the external fluctuating signal by the single protein channel and is not influenced by other noise sources due to the electrical equipment. The top curve in Fig. 3d emphasizes the importance of the rectification shown in Fig. 1b and d: when the protein exhibits quasi-ohmic behavior, the charging process is noticeably reduced. As to the charging process efficiency, it can be estimated from the energy ratio:2
 | (1) |
By assuming that the channel is a potential dependent resistor connected in series with the capacitor, the charging process can be simulated. When the circuit is fed with an oscillating potential of amplitude V0, the charging process is described by the equation
| dVC(t)/dt = Iout(t)/C | (2) |
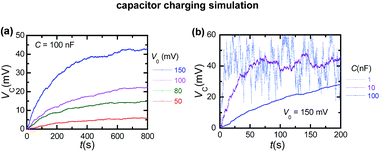 | ||
| Fig. 4 (a) Time traces for the simulated capacitor voltage VC(t) during the charging process with C = 100 nF. The curves are parametric in the amplitude V0 of the oscillating potential. (b) Simulated charging curves for the capacitances in Fig. 3c and V0 = 150 mV. | ||
Fig. 5a (experiment) and Fig. 5b (simulation) show the frequency response of the capacitor charging curve for the case of a sinusoidal input voltage. The capacitor voltage oscillates at low frequencies because of the increased coupling with the input signal compared with the high frequency case. The theoretical simulations can approximately describe the observed results.
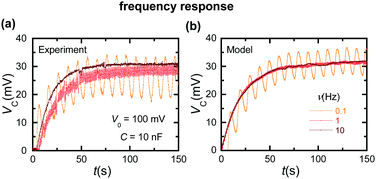 | ||
| Fig. 5 (a) The experimental capacitor charging curves for sinusoidal input voltages of frequencies 0.1, 1, and 10 Hz with C = 10 nF and V0 = 100 mV. (b) The simulation curves. | ||
Taken together, Fig. 2–5 demonstrate that the electrical rectification provided by a single ion channel inserted in a lipid bilayer is able to convert zero time-average signals into directional currents which allow charging of a conventional electronic element. These results must not be extrapolated to the single cell case because of the increased complexity involved in this change of scale and the multitude of ion channels present in the biological membrane.1
However, the present experimental approach should have wide interest: the protein ion channels constitute the building blocks of the cell membrane electrical network and are also involved in the design of ionic circuits for bio-electronic interfaces and nanofluidic sensors. While external amplitudes of tens of millivolts may be larger than those likely to arise from most environmental sources, they are typical of the electrical signals found in the cell cycle16 and intercellular communication,17 bio-nanoelectronic interfaces,24 bioinspired sensing nanochannels,5,6,25 and bio-electrochemical cells.26 Note also that the artificial nanostructures should be effectively coupled to conventional electronic elements such as capacitors to achieve full functionality and short-time responses.
4. Conclusions
Oscillating fields and membrane potentials are of interest to biophysical processes based on protein ion channels.16–18 A protein pore with an asymmetric charge distribution constitutes a soft matter nanostructure which can mimic the electrical characteristics of macroscopic solid-state diodes.27 By rectifying randomly fluctuating signals of amplitudes in the range of tens of millivolts, this biological pore permits charging of a commercial capacitor to significant voltages within minutes. The protein channel is inserted in the lipid bilayer characteristic of cell membranes and operates in aqueous salt solutions.1,17 The described experimental approach suggests new methods to quantify the signal averaging of low frequency oscillating signals at a more fundamental level than the traditional cell and tissue scales.14There are different, commercially available ion channels and toxin pore-formers (e.g. gramicidin A and alpha-hemolysin). The results show an efficient coupling between these biological nanostructures and conventional capacitors. This coupling can be of interest for the design of signal averaging and energy transduction schemes using bio-electronic interfaces and nanopore-based ionic circuits where miniaturization leads to novel properties.5–9 Also, the significant charging effects observed suggest that noisy zero-average signals could produce noticeable accumulative results in highly rectifying ion channels.
Acknowledgements
We acknowledge the support from the Ministry of Economic Affairs and Competitiveness and FEDER (project MAT2015-65011-P) and the Generalitat Valenciana (project Prometeo/GV/0069). We thank Profs. A. Alcaraz and V. M. Aguilella for fruitful suggestions. This paper is devoted to the memory of Professor Kyösti Kontturi, Aalto University, Finland.Notes and references
- B. Hille, Ionic Channels of Excitable Membranes, Sinauer, Sunderland, 1992 Search PubMed.
- V. Gomez, P. Ramirez, J. Cervera, S. Nasir, M. Ali, W. Ensinger and S. Mafe, Sci. Rep., 2015, 5, 9501–9505 CrossRef CAS PubMed.
- Z. Siwy and A. Fulinski, Phys. Rev. Lett., 2002, 89, 198103 CrossRef CAS PubMed.
- M. Queralt-Martín, M. E. García-Giménez, V. M. Aguilella, P. Ramirez, S. Mafe and A. Alcaraz, Appl. Phys. Lett., 2013, 103, 043707 CrossRef.
- K. Xiao, L. Wen and L. Jiang, Small, 2016, 3, 3339–3342 Search PubMed.
- Q. Liu, L. Wen, K. Xiao, H. Lu, Z. Zhang, G. Xie, X.-Y. Kong, Z. Bo and L. Jiang, Adv. Mater., 2016, 28, 3181–3186 CrossRef CAS PubMed.
- N. Misra, J. A. Martinez, S.-C. J. Huang, Y. Wang, P. Stroeve, C. P. Grigoropoulos and A. Noy, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13780–13784 CrossRef CAS PubMed.
- P. Ramirez, M. Ali, W. Ensinger and S. Mafe, Appl. Phys. Lett., 2012, 101, 133108 CrossRef.
- K. Tybrandt, R. Forchheimer and M. Berggren, Nat. Commun., 2012, 3, 871 CrossRef PubMed.
- V. Gomez, J. Cervera, S. Nasir, M. Ali, W. Ensinger, S. Mafe and P. Ramirez, Electrochem. Commun., 2016, 62, 29–33 CrossRef CAS.
- W. Guo, L. Cao, J. Xia, F.-Q. Nie, W. Ma, J. Xue, Y. Song, D. Zhu, Y. Wang and L. Jiang, Adv. Funct. Mater., 2010, 20, 1339–1344 CrossRef CAS.
- J. Gao, W. Guo, D. Feng, H. Wang, D. Zhao and L. Jiang, J. Am. Chem. Soc., 2014, 136, 12265–12272 CrossRef CAS PubMed.
- W. Guo, Y. Tian and L. Jiang, Acc. Chem. Res., 2013, 12, 2834–2846 CrossRef PubMed.
- M. Hronik-Tupaj and D. L. Kaplan, Tissue Eng., Part B, 2012, 18, 167–180 CrossRef CAS PubMed.
- D. J. Blackiston, K. A. McLaughlin and M. Levin, Cell Cycle, 2009, 8, 3527–3536 CrossRef CAS PubMed.
- M. Levin, BioEssays, 2012, 34, 205–217 CrossRef CAS PubMed.
- F. Chang and N. Minc, Annu. Rev. Cell Dev. Biol., 2014, 30, 317–336 CrossRef CAS PubMed.
- J. Cervera, J. A. Manzanares and S. Mafe, J. Phys. Chem. B, 2015, 119, 2968–2978 CrossRef CAS PubMed.
- B. T. Chernet and M. Levin, J. Clin. Exp. Oncol., 2013,(suppl 1), S1–002 Search PubMed.
- M. Levin, Wiley Interdiscip. Rev.: Syst. Biol. Med., 2013, 5, 657–676 CrossRef CAS PubMed.
- A. Alcaraz, E. M. Nestorovich, M. Aguilella-Arzo, V. M. Aguilella and S. M. Bezrukov, Biophys. J., 2004, 87, 943–957 CrossRef CAS PubMed.
- A. Alcaraz, P. Ramírez, E. García-Giménez, M. L. López, A. Andrio and V. M. Aguilella, J. Phys. Chem. B, 2006, 110, 21205–21209 CrossRef CAS PubMed.
- C. Verdiá-Báguena, M. Queralt-Martín, V. M. Aguilella and A. Alcaraz, J. Phys. Chem. C, 2012, 116, 6537–6542 Search PubMed.
- A. Noy, Adv. Mater., 2011, 23, 807–820 CrossRef CAS PubMed.
- M. Ali, S. Nasir, P. Ramirez, J. Cervera, S. Mafe and W. Ensinger, J. Phys. Chem. C, 2013, 117, 18234–18242 CAS.
- O. Yehezkeli, R. Tel-Vered, J. Wasserman, A. Trifonov, D. Michaeli, R. Nechushtai and I. Willner, Nat. Commun., 2012, 3, 742 CrossRef PubMed.
- I. Vlassiouk and Z. S. Siwy, Nano Lett., 2007, 7, 552–556 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2017 |


