Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the origin of the great rigidity of self-assembled diphenylalanine nanotubes
Pavel
Zelenovskiy
*a,
Igor
Kornev
b,
Semen
Vasilev
a and
Andrei
Kholkin
ac
aInstitute of Natural Sciences and Mathematics, Ural Federal University, Ekaterinburg, 620000, Russia. E-mail: zelenovskiy@urfu.ru
bSPMS Laboratory, Ecole Centrale Paris, Chatenay-Malabry, 92295, France
cPhysics Department & CICECO – Aveiro Institute of Materials, University of Aveiro, Aveiro, 3810-193, Portugal
First published on 13th October 2016
Abstract
The elastic properties of the nanotubes of self-assembled aromatic dipeptide diphenylalanine are investigated by means of Raman spectroscopy and a mass-in-mass 1D model. Analysis of nanotubes' lattice vibrations reveals the essential contribution of the water in the nanochannel core of the tubes to the Young’s modulus and high water mobility along the channel. Direct measurements of the Young’s modulus performed by nanoindentation confirm the obtained results.
Self-assembly of bioorganic materials is a convenient tool for the fabrication of functional micro- and nanodevices with outstanding properties.1 Peptides are of particular importance as molecular building blocks because of their unique characteristics that can be tuned by changing the amino acid sequence and conjugating chemical groups to achieve better functionality.2,3 Assembly mechanisms based on various noncovalent intermolecular interactions4 allow peptides to readily adopt diverse 3D architectures such as vesicles, micelles, monolayers, bilayers, fibers, tubes, ribbons, spheres, and tapes.5–7
Recently, nanotubes of short aromatic peptides (namely, diphenylalanine, FF, consisting of two molecules of amino acid phenylalanine, F) have attracted significant attention, due to their outstanding physical and chemical properties, which are interesting from both the fundamental and applied points of view.4–8 Along with inherent biocompatibility, they possess high aspect ratios,9 strong piezoelectricity,10,11 ferroelectricity,12,13 and interesting optical properties related to quantum confinement of electrons and holes.8,14 These useful functional properties are considered to be important for the design of novel biosensors and bioelectronic and biomolecular devices.7,8 For example, it has been shown that microtubes of FF (self-assembled bundles of the nanotubes) exhibit clear piezoelectric resonance at MHz frequencies with high enough quality factor and can thus serve as piezoelectric sensors and actuators in micromechanical systems (MEMS).10,15,16
Special attention was paid to the remarkably rigid structure of FF nanotubes. Its experimentally measured transversal Young’s modulus varies from 19 to 27 GPa,17,18 and is several times larger than that of other peptide fibrils (about 3.3 GPa19). An attempt to shed light on the origin of such enormous stiffness was made using first-principles calculations.20 However, the obtained value of the Young’s modulus was still less than a half of the experimentally observed one, even when using the Tkatchenko–Scheffler (TS) correction, which allowed describing accurately hydrogen and van der Waals bonding, essential for biomolecular crystals.20
In our opinion, the main reason for such divergence is neglecting the water in the nanochannel core of the nanotubes. Its presence there was confirmed by X-ray analysis,21 photoluminescence,22 and Raman measurements.23,24 Typically, FF nanotubes are produced from water rich solution, and water plays a crucial role in the nanotube formation.25–27 Each FF monomer creates hydrogen bonds with water molecules,21 which may thus contribute to the mechanical properties of the nanotubes. Exceptionally high values of Young’s moduli (up to 44 GPa) due to hydrogen bonds between molecules were observed in several amino acid crystals.28 A similar effect should be expected for the FF nanotubes.
In this work we developed a simple mechanical model of FF nanotubes taking into account the existence of water in the nanochannel core of the tubes. Analysis of nanotubes' lattice vibrations by Raman spectroscopy in the context of the proposed model allowed us to calculate effective elastic constants and to demonstrate the large contribution of water to the tubes' Young’s moduli.
It is known that FF monomers in water-rich solution form helical nanotubes filled with water molecules via a self-assembly process.21,23 Water molecules are held in the nanochannel core by radially oriented hydrogen bonds with FF monomers, whereas the latter interact among themselves via both hydrogen bonds and van der Waals forces.21,29
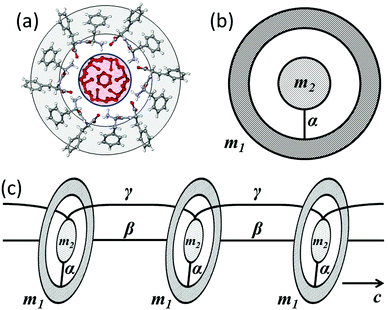
The unit cell of the nanotube (one step of the helix) consists of two embedded interacting subsystems: an FF ring (6 monomers) and water (24 molecules, 4 molecules per FF monomer22) inside the ring (Fig. 1(a)). Such systems can be described by the equivalent mass-in-mass unit of the same size (Fig. 1(b)). The masses of the FF ring and of its water “core” are m1 = 3.11 × 10−24 kg and m2 = 0.72 × 10−24 kg, respectively. Interaction between the FF ring and the water subsystem for the X and Y directions is characterized by the same effective spring constant α. The sequence of aligned unit cells forms the nanotube (Fig. 1(c)). Longitudinal interaction between adjacent rings and water therein occurs with effective spring constants β and γ, respectively. Individual nanotubes bond together by aromatic rings and form microtubes, which can be considered as a hexagonal crystal.23,29
The equations of motion for this system provide dispersive relations in the general form:
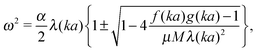 | (1) |
It is worth noting that for k = 0 eqn (1) is reduced to a simple expression for vibrations of crystal with two atoms per primitive cell.30 Therefore, keeping in mind the application to Raman scattering, the frequency of the optical mode at the center of the Brillouin zone is:30
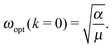 | (2) |
This expression can be used for determination of the spring constant α, assuming ωopt to be an effective frequency of lattice vibrations measured by Raman spectroscopy. The obtained result will depend on the polarization conditions of the measurements, and therefore different spring constants αij relating to lattice vibrations in different directions in the nanotube can be obtained.
The effective elastic constants of FF nanotubes can be derived from the analysis of the Raman spectra. A comparison of the frequencies of acoustic vibrations obtained in the context of the used model with those calculated in continuous approximation gives a relation between microscopic spring constants αij and macroscopic elastic constants Cij:30
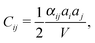 | (3) |
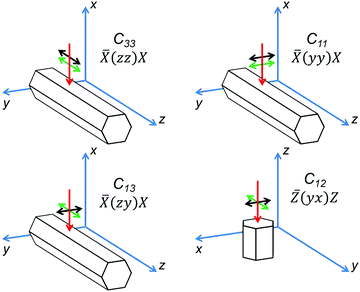
For hexagonal crystals, the elastic constants form a tensor with five independent components (Voigt notations are used):31C11, C12, C13, C33 and C44. In the most common backscattering geometry of Raman measurements, a variation of the polarization direction allows determination of 4 components of the effective elastic tensor (Fig. 2). For ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (zz)X and
(zz)X and ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (yy)X geometries, where polarization of a laser beam is parallel to the analyzer's orientation and is oriented either along or perpendicular to the tube axis, eqn (2) and (3) provide C33 and C11 constants, respectively. In the
(yy)X geometries, where polarization of a laser beam is parallel to the analyzer's orientation and is oriented either along or perpendicular to the tube axis, eqn (2) and (3) provide C33 and C11 constants, respectively. In the ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (zy)X geometry the polarizations of the laser beam and the analyzer are 90°-crossed; therefore the constant C13 can be found. For estimation of the C12 constant the spectrum has to be measured on the end face of the tube in the
(zy)X geometry the polarizations of the laser beam and the analyzer are 90°-crossed; therefore the constant C13 can be found. For estimation of the C12 constant the spectrum has to be measured on the end face of the tube in the ![[Z with combining macron]](https://www.rsc.org/images/entities/i_char_005a_0304.gif) (yx)Z geometry. The C44 constant cannot be determined by Raman spectroscopy. Raman spectra of all four geometries are presented in Fig. 3.
(yx)Z geometry. The C44 constant cannot be determined by Raman spectroscopy. Raman spectra of all four geometries are presented in Fig. 3.
For estimation of effective elastic constants we used sufficiently long FF microtubes grown from water rich solution using a common procedure.9,25 Raman spectra were measured using the confocal Raman microscope Alpha300AR (WITec GmbH, Germany) with an excitation wavelength of 488 nm and a spectral resolution of about 3 cm−1.
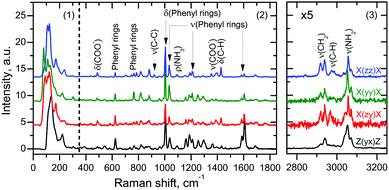
A typical Raman spectrum of FF microtubes consists of three separate regions: region 1 (from 10 to 375 cm−1) corresponds to lattice vibrations, whereas region 2 (from 375 to 1850 cm−1) and region 3 (from 2750 to 3250 cm−1) are due to vibrations of various functional groups (Fig. 3). Assignment of high-frequency spectral lines can be based on the analysis of published data,32 whereas detailed analysis of low-frequency lines is hampered by a rather complicated tube structure and highly overlapping spectral lines. Deconvolution of this region into separate lines provides little information about the tube structure, regardless of the fact that the assignment of several low-frequency lines has been performed recently.24
The effective frequency of lattice vibrations can be calculated either by fitting the low-frequency spectral region by a single curve (Lorentzian or Gaussian) or by calculating the weighted average over this region. The second method is more precise since it takes into account low intensive lines, whereas fitting by a single curve mainly gives the maximum frequency in the region.
The calculation of the weighted average over the low-frequency region of Raman spectra (Fig. 3) gives average values of the effective frequency 131.2 and 126.8 cm−1 for ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (zz)X and
(zz)X and ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (yy)X geometries, respectively, and 134.8 and 141.9 cm−1 for
(yy)X geometries, respectively, and 134.8 and 141.9 cm−1 for ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) (zy)X and
(zy)X and ![[Z with combining macron]](https://www.rsc.org/images/entities/i_char_005a_0304.gif) (yx)Z geometries, respectively.
(yx)Z geometries, respectively.
It is worth noting that edge filters, which are usually used in spectroscopic systems, cut part of the low-frequency region up to 50–100 cm−1. However, this region is usually overlapped with a broad boson peak and shoulders of the Rayleigh line, and therefore information about the lattice vibrations here cannot be extracted anyway. We found that the spectral resolution of our instrument has little effect on the resulting elastic constants and even 30 cm−1 variation of the upper and lower limits of region 1 changes ET and EL by less than 0.3 GPa.
Thus, using the measured effective frequencies and estimated reduced mass of the ring, four independent components of effective elastic tensor CWij of the water-filled nanotubes can be calculated. The obtained values are in line with those found in ref. 20 within a density functional theory calculation using the Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation for the exchange–correlation (Table 1).
| C FF ij | C W ij | C tot ij | ||
|---|---|---|---|---|
| PBE | PBE+TS | |||
| C 11 | 5.29 | 17.56 | 9.17 | 26.73 |
| C 12 | 2.18 | 11.91 | 2.60 | 14.51 |
| C 13 | 2.34 | 11.00 | 2.35 | 13.35 |
| C 33 | 14.08 | 24.05 | 0.50 | 24.55 |
The values of C12 and C13 are close to those calculated in ref. 20 for empty tubes (Table 1), whereas a higher value of C11 demonstrates the pronounced effect of the water subsystem on the elasticity of the tube in a transversal direction. The low value of C33 shows that water molecules are weakly bound to FF rings and can easily move along the nanochannel, thus making FF tubes interesting for various micro- and nanofluidic applications.33 At the same time, the longitudinal mobility of water has only a negligible effect on the longitudinal stiffness of the tube, which is therefore determined by the interaction between FF monomers in the adjacent rings only. This interaction cannot be taken into account in our model, since eqn (2) for the effective frequency of the lattice vibrations does not include the spring constant β.
To take into account both water and FF contributions, the total elastic constants of the FF nanotubes can be calculated: Ctotij = CWij + CFFij. Here CWij describes water contribution and is found in this work in the context of the proposed model. For CFFij we used elastic constants calculated in ref. 20 in the “PBE+TS” approach, i.e. with the van der Waals interactions after the Tkatchenko–Scheffler scheme included on top of the PBE exchange-correlation. The inclusion of this interaction is essential for accurately describing the long-range interaction between FF rings. The obtained values of the total elastic constants are presented in Table 1.
The hardness of the FF microtubes can be described by transversal ET (across the tube) and longitudinal EL (along the tube) Young’s moduli due to the anisotropy of the microtubes. ET and EL can be written in terms of elastic constants:34
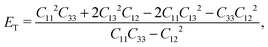 | (4) |
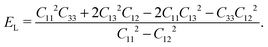 | (5) |
However, the obtained value (16.77 GPa) is far though from another experimental value (27 GPa).17 This experimental value was obtained by a bending beam model using an atomic force microscope (AFM) on nanotubes suspended across the microcavities. However, this method provides results strongly depending on the nanotube fixed at the edges of the cavity.35,36
To get more precise values of the transversal Young’s modulus, we performed direct measurements of ET by the dynamical nanoindentation method. The nano-hardness tester NanoScan-4D (FSBI TISNCM, Russia) equipped with a Berkovich indenter (diamond pyramid) was used. The values of ET were determined from loading–unloading curves by the Oliver–Pharr method.37 Measurements were performed in more than 50 points of several FF nanotubes fixed at the solid substrate. Our tests showed an instrumental error less than 2%.
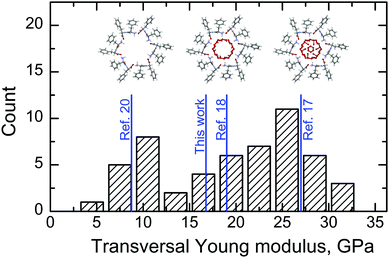
Two characteristic values of ET were revealed (see Table 2 and Fig. 4). The first value (10 GPa) is close to that obtained from first-principles modeling of empty nanotubes.20 The second value (25 GPa) is close to that measured in ref. 17. The dispersion of these characteristic values is larger than the instrumental error, reproducible and can be attributed to inhomogeneous filling of the nanotubes due to the high mobility of water molecules along the nanochannel.38,39 In this case the lowest values of the Young’s modulus correspond to almost empty nanotubes, whereas completely filled tubes provide the largest ET (see insets in Fig. 4). It is worth noting that only a part of the water molecules in FF tubes is considered in our model. This corresponds to partially filled nanotubes, and therefore the value of ET calculated in the present work falls into the intermediate range (Fig. 4). Additional water molecules that can exist in the nanochannel core40,41 introduce additional stiffness into the nanotube, and therefore higher values of Young’s moduli can be observed. The highest observed value of ET is about 31 GPa (Fig. 4).
Finally, the value of EL calculated in this work is very close to that obtained in ref. 20 in PBE–TS approximation, since movable water molecules weakly contribute to longitudinal stiffness. Experimental measurements of this value are conjugated with high technical difficulties and are still to be done.
In summary, the essential contribution of water molecules inside the nanochannel of diphenylalanine peptide nanotubes to its transversal Young’s modulus is demonstrated. Analysis of the low-frequency region of the Raman spectra of the nanotubes in the context of a mass-in-mass 1D model allowed refining four independent constants in the effective elastic tensor and to demonstrate the high mobility of water molecules along the nanochannels. Direct measurements of the transversal Young’s modulus using a nanoindenter showed that its value strongly depends on the degree of nanotube filling by water, whereas the longitudinal Young’s modulus is primarily determined by the interaction between peptide monomers.
The equipment of the Ural Center for Shared Use “Modern nanotechnology” UrFU was used. The research was made possible by the President of Russian Federation grant for young scientists (Contract 14.Y30.15.6554-MK), by the Government of the Russian Federation (Act 211, Agreement 02.A03.21.0006), and by the Ministry of Foreign Affairs of France within Metchnikov Scholarship (grant 851365A). Part of this work was developed in the scope of Project CICECO-Aveiro Institute of Materials (ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and, when applicable, cofinanced by FEDER under the PT2020 Partnership Agreement.
References
- J.-M. Lehn, Science, 2002, 295, 2400–2403 CrossRef CAS PubMed.
- C.-L. Chen and N. Rosi, Angew. Chem., Int. Ed., 2010, 49, 1924–1942 CrossRef CAS PubMed.
- C. A. E. Hauser and S. Zhang, Chem. Soc. Rev., 2010, 39, 2780–2790 RSC.
- S. Cavalli, F. Albericio and A. Kros, Chem. Soc. Rev., 2010, 39, 241–263 RSC.
- E. Gazit, Chem. Soc. Rev., 2007, 36, 1263–1269 RSC.
- G. Rosenman, P. Beker, I. Koren, M. Yevnin, B. Bank-Srour, E. Mishina and S. Semin, J. Pept. Sci., 2011, 17, 75–87 CrossRef CAS PubMed.
- L. Adler-Abramovich and E. Gazit, Chem. Soc. Rev., 2014, 43, 6881–6893 RSC.
- D. Bonetti, A. Toto, R. Giri, A. Morrone, D. Sanfelice, A. Pastore, P. Temussi, S. Gianni and M. Brunori, Phys. Chem. Chem. Phys., 2014, 16, 6391–6397 RSC.
- A. Nuraeva, S. Vasilev, D. Vasileva, P. Zelenovskiy, D. Chezganov, A. Esin, S. Kopyl, K. Romanyuk, V. Y. Shur and A. L. Kholkin, Cryst. Growth Des., 2016, 16, 1472–1479 CAS.
- A. Kholkin, N. Amdursky, I. Bdikin, E. Gazit and G. Rosenman, ACS Nano, 2010, 4, 610–614 CrossRef CAS PubMed.
- S. Vasilev, P. Zelenovskiy, D. Vasileva, A. Nuraeva, V. Y. Shur and A. L. Kholkin, J. Phys. Chem. Solids, 2016, 93, 68–72 CrossRef CAS.
- Z. Gan, X. Wu, X. Zhu and J. Shen, Angew. Chem., Int. Ed., 2013, 52, 2055–2059 CrossRef CAS PubMed.
- I. Bdikin, V. Bystrov, S. Kopyl, R. P. G. Lopes, I. Delgadillo, J. Gracio, E. Mishina, A. Sigov and A. L. Kholkin, Appl. Phys. Lett., 2012, 100, 043702 CrossRef.
- N. Amdursky, M. Molotskii, D. Aronov, L. Adler-Abramovich, E. Gazit and G. Rosenman, Nano Lett., 2009, 9, 3111–3115 CrossRef CAS PubMed.
- R. de la Rica, C. Pejoux and H. Matsui, Adv. Funct. Mater., 2011, 21, 1018–1026 CrossRef CAS PubMed.
- E. D. Bosne, A. Heredia, S. Kopyl, D. V. Karpinsky, A. G. Pinto and A. L. Kholkin, Appl. Phys. Lett., 2013, 102, 073504 CrossRef.
- L. Niu, X. Chen, S. Allen and S. J. B. Tendler, Langmuir, 2007, 23, 7443–7446 CrossRef CAS PubMed.
- N. Kol, L. Adler-Abramovich, D. Barlam, R. Z. Shneck, E. Gazit and I. Rousso, Nano Lett., 2005, 5, 1343–1346 CrossRef CAS PubMed.
- J. F. Smith, T. P. J. Knowles, C. M. Dobson, C. E. MacPhee and M. E. Welland, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15806–15811 CrossRef CAS PubMed.
- I. Azuri, L. Adler-Abramovich, E. Gazit, O. Hod and L. Kronik, J. Am. Chem. Soc., 2014, 136, 963–969 CrossRef CAS PubMed.
- J. Kim, T. H. Han, Y.-I. Kim, J. S. Park, J. Choi, D. G. Churchill, S. O. Kim and H. Ihee, Adv. Mater., 2010, 22, 583–587 CrossRef CAS PubMed.
- M. Wang, S. Xiong, X. Wu and P. K. Chu, Small, 2011, 7, 2801–2807 CrossRef CAS PubMed.
- X. Wu, S. Xiong, M. Wang, J. Shen and P. K. Chu, J. Phys. Chem. C, 2012, 116, 9793–9799 CAS.
- X. Wu, S. Xiong, M. Wang, J. Shen and P. K. Chu, Opt. Express, 2012, 20, 5119–5126 CrossRef CAS PubMed.
- P. S. Zelenovskiy, V. Y. Shur, A. S. Nuraeva, S. G. Vasilev, D. S. Vasileva, D. O. Alikin, D. S. Chezganov, V. P. Krasnov and A. L. Kholkin, Ferroelectrics, 2015, 475, 127–134 CrossRef CAS.
- M. I. Souza, E. R. Silva, Y. M. Jaques, F. F. Ferreira, E. E. Fileti and W. A. Alves, J. Pept. Sci., 2014, 20, 554–562 CrossRef CAS PubMed.
- T. D. Do and M. T. Bowers, Anal. Chem., 2015, 87, 4245–4252 CrossRef CAS PubMed.
- I. Azuri, E. Meirzadeh, D. Ehre, S. R. Cohen, A. M. Rappe, M. Lahav, I. Lubomirsky and L. Kronik, Angew. Chem., Int. Ed., 2015, 54, 13566–13570 CrossRef CAS PubMed.
- C. H. Gorbitz, Chem. Commun., 2006, 2332–2334 RSC.
- C. Kittel, Introduction to Solid State Physics, John Wiley & Sons, New York, 1953 Search PubMed.
- J. F. Nye, Physical Properties of Crystals: Their Representation by Tensors and Matrices, Oxford University Press, Oxford, NY, 1957 Search PubMed.
- B. Hernandez, F. Pfluger, S. G. Kruglik and M. Ghomi, J. Raman Spectrosc., 2013, 44, 827–833 CrossRef CAS.
- N. B. Sopher, Z. R. Abrams, M. Reches, E. Gazit and Y. Hanein, J. Micromech. Microeng., 2007, 17, 2360 CrossRef CAS.
- A. F. Bower, Applied Mechanics of Solids, CRC Press, Boca Raton, FL, 2009 Search PubMed.
- Y. Chen, B. L. Dorgan, D. N. McIlroy and E. D. Aston, J. Appl. Phys., 2006, 100, 104301 CrossRef.
- D. Kluge, F. Abraham, S. Schmidt, H.-W. Schmidt and A. Fery, Langmuir, 2010, 26, 3020 CrossRef CAS PubMed.
- W. Oliver and G. Pharr, J. Mater. Res., 1992, 7, 1564–1583 CrossRef CAS.
- J. Comer, F. Dehez, W. Cai and C. Chipot, J. Phys. Chem. C, 2013, 117, 26797 CAS.
- L. Ruiz, Y. Wu and S. Keten, Nanoscale, 2015, 7, 121 RSC.
- C. H. Gorbitz, Chem. – Eur. J., 2001, 7, 5153 CrossRef CAS.
- T. Andrade-Filho, T. C. Martins, F. F. Ferreira, W. A. Alves and A. R. Rocha, Theor. Chem. Acc., 2016, 135, 185 CrossRef.
| This journal is © the Owner Societies 2016 |