Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Investigating lithium-ion battery materials during overcharge-induced thermal runaway: an operando and multi-scale X-ray CT study†
Donal P.
Finegan
a,
Mario
Scheel
bc,
James B.
Robinson
a,
Bernhard
Tjaden
a,
Marco
Di Michiel
b,
Gareth
Hinds
d,
Dan J. L.
Brett
a and
Paul R.
Shearing
*a
aElectrochemical Innovation Lab, Department of Chemical Engineering, University College London, Torrington Place, London WC1E 7JE, UK. E-mail: p.shearing@ucl.ac.uk
bESRF, The European Synchrotron, 71 Rue des Martyrs, 38000 Grenoble, France
cSynchrotron Soleil, L'Orme des Merisiers, 91190 Saint-Aubin, France
dNational Physical Laboratory, Hampton Road, Teddington, Middlesex TW11 0LW, UK
First published on 21st June 2016
Abstract
Catastrophic failure of lithium-ion batteries occurs across multiple length scales and over very short time periods. A combination of high-speed operando tomography, thermal imaging and electrochemical measurements is used to probe the degradation mechanisms leading up to overcharge-induced thermal runaway of a LiCoO2 pouch cell, through its interrelated dynamic structural, thermal and electrical responses. Failure mechanisms across multiple length scales are explored using a post-mortem multi-scale tomography approach, revealing significant morphological and phase changes in the LiCoO2 electrode microstructure and location dependent degradation. This combined operando and multi-scale X-ray computed tomography (CT) technique is demonstrated as a comprehensive approach to understanding battery degradation and failure.
Introduction
Lithium-ion batteries are ubiquitous energy storage devices in portable electronics owing to their high energy and power densities. The synergy between lithium-ion batteries and clean, renewable energy sources to achieve global CO2 emission targets also makes them the technology of choice in the rapidly expanding market for electric and hybrid electric vehicles. However, for many advanced applications, lithium-ion batteries are required to operate safely over a range of temperatures and during high charge and discharge rates. Although failure is rare the consequences are severe and several well publicised incidents1–3 involving catastrophic battery failures have highlighted the need to further reduce the risk associated with battery packs and modules.4,5Overcharge of lithium-ion batteries poses a significant safety risk as chemical and electrochemical reactions can occur between cell materials, and is one of the primary causes of thermal runaway, as seen in the recent spate of failures associated with charging self-balancing scooters; consequently, overcharge tests have been integrated into safety test standards for lithium-ion batteries.6,7 The magnitude of the energy released during failure increases with increasing state-of-charge (SOC), as the chemical energy stored in the electrode materials is released, hence thermal runaway resulting from overcharge is particularly catastrophic.8
At a critical temperature, thermal runaway takes place when a chain of exothermic reactions between the electrodes and electrolyte occurs. For example, the LiCoO2 positive electrode undergoes exothermic decomposition at elevated temperatures through a series of reduction steps in the presence of electrolyte.9,10 There have been numerous studies focusing on the reaction pathways of positive electrode materials during failure,10–13 but a spatial and temporal understanding of the reaction processes and their effects on particle microstructure, rate of reaction and critical temperatures reached during failure, is yet to be achieved.
It is well known that the microstructure of electrode materials plays an important role in determining the performance of cells,14,15 but the role of particle morphology in the safety of cells is not yet well understood. Jiang and Dahn16 used accelerated rate calorimetry to investigate the effect of particle size on the onset temperature of thermal runaway, demonstrating that a larger particle size is more thermally stable. More recently, Geder et al.17 demonstrated through thermogravimetric analyses that the mass loss associated with the decomposition of LiCoO2 increases linearly with surface area at 400 °C. A higher mass loss corresponds to increased heat generation during failure, and increased gas (including O2) evolution, further fuelling decomposition of the electrolyte;18 smaller particles were shown to release more oxygen and have an oxygen deficiency at their surface.
The fast reaction kinetics between the electrolyte and the positive electrode lead to generation of heat at a very high rate, which can result in internal temperatures of commercial cells reaching in excess of 1000 °C,19 damaging and by-passing integrated safety devices,20,21 and posing a great risk to modular systems where cell-to-cell propagation of failure is a major concern.22 Thus, the reliability and safety of lithium-ion batteries is considered a function of the entirety of the cell or modular system. Understanding the link between behaviour on the microscale and the catastrophic effects observed for full cells and modular systems is imperative to progress towards safer battery designs. With the advancement of operando X-ray imaging techniques, tracking the propagation of failure at high frame rates and across multiple length scales is now possible.19,23–25 X-ray computed tomography (CT) has previously been used to perform multi-length scale analyses of battery materials,26 and has also been demonstrated as an effective diagnostic tool for lithium-ion batteries during,19,24 and after27 failure.
Here, we combine high-speed operando synchrotron X-ray CT with thermal and electrochemical measurements to link, and assess, the observed morphological, thermal and electrical response of a commercial LiCoO2 battery during overcharge-induced failure. A multi-scale comparative analysis of the cell in its fresh and failed state is also performed to elucidate failure mechanisms across multiple length scales, from individual electrode particles to the full cell architecture. Image-based quantification is used to determine the change in electrode particle morphology via a particle size distribution analysis, and the effects of particle morphology on the failure mechanisms of lithium-ion batteries are discussed.
Experimental
Electrical abuse testing and operando synchrotron CT
Fast tomographic imaging was performed at beamline ID15A at The European Synchrotron (ESRF).28,29 An in situ containment system was designed to allow simultaneous high speed X-ray CT and thermal imaging (Fig. 1a) and is discussed in more detail in a previous work.19 The overcharge abuse test was performed on a Turnigy nanotech 160 mA h lithium-ion pouch cell (dimensions 39 mm × 12 × 8 mm) which consists of a LiCoO2 positive electrode, polymer separator and graphite negative electrode. The Turnigy cell is designed to cater for high charge (10C) and discharge (30C) rates (where a C-rate is the rate at which a battery is discharged relative to its maximum capacity). In this study the battery was overcharged from 100% SOC (4.2 V) at a constant current of 3 A (18.75 C) until failure, which is well outside the safe operating limits recommended by manufacturers.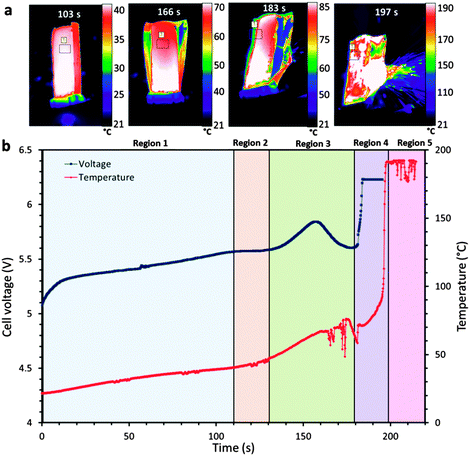 | ||
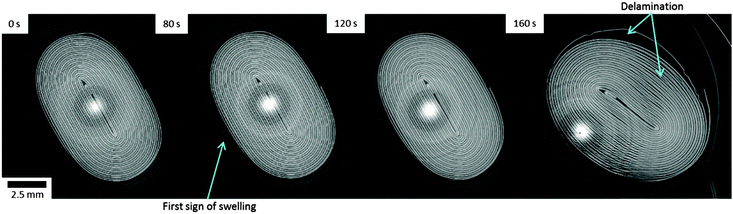
| Fig. 1 (a) Sequence of time-stamped thermal images showing the increasing surface temperature of the LiCoO2 pouch cell leading up to thermal runaway. The average temperature within the labelled square is plotted. (b) Plot of the cell voltage and surface temperature, leading up to and during thermal runaway. The battery was charged at a constant current of 3 A (18.75 C), from 100% SOC (4.2 V) until failure. Real time thermal images are presented in Movie S1 (ESI†). | ||
Use of an electrical slip ring (P4 + Compact Slip Ring, Moog, UK) built into the ID15A rotation stage (ABR1000, Aerotech, USA) allowed simultaneous rotation of the sample (for X-ray CT) and electrical charging.19 The cell voltage plot during overcharge is shown alongside the surface temperature profile in Fig. 1b; the temperature presented in the plot was taken as an average over an area ca. 9 mm2 on the surface of the cell (shown in Fig. 1a).
The surface temperature of the battery was recorded using a thermal camera (FLIR SC5000MB, FLIR Systems, France). The thermal camera was set to the calibrated range of 15 °C to 200 °C during these experiments. A uniform layer of high emissivity black paint (with a calibrated emissivity of 0.96 over the range 40–180 °C) was applied to the surface of the battery before imaging. The FLIR SC5000MB thermal camera has an extended wavelength detector to capture infra-red wavelengths between 2.5 μm and 7 μm. In the calibrated range, the camera has a noise equivalent temperature difference <20 mK. The manufacturer specifies a measurement accuracy of ±1 °C or ±1% of the temperature in degrees Celsius. Images were recorded at 25 Hz and the real time movie is provided as Movie S1 (ESI†).
The Turnigy cell was imaged using a 76 keV monochromatic synchrotron X-ray beam. The X-ray absorption signal was converted into a visible light signal by a LuAG:Ce scintillator screen and then collected by a 1× macro objective. A high-speed PCO Dimax CMOS camera (PCO AG, Germany) was used to record the images. The field of view (FOV) was 10.5 mm × 7.6 mm, which consisted of 960 × 700 pixels (horizontal × vertical) and gave a pixel resolution of 10.9 μm. The sample rotation axis was at the edge of the FOV, such that through a 360° rotation it was possible to double the size of the 3D image in the horizontal direction.30 The resulting 3D reconstruction was then 1785 × 1785 × 700 pixels (19.2 mm × 19.2 mm × 7.6 mm). Each tomogram consisted of 2 × 2000 half projections using an exposure time of 0.7 ms. The acquisition time for each tomogram was 2.8 s and one tomogram was captured every 40 s.
Multi-scale laboratory X-ray micro-CT
Using material extracted from the same Turnigy cell imaged in the synchrotron, tomographic reconstructions of varying sample size and resolution were produced using lab-based X-ray CT systems (Zeiss Xradia Versa 520 and Zeiss Xradia Ultra 810, Carl Zeiss XRM, Pleasanton, CA, USA). The specific imaging properties for each scan are provided as ESI.† Materials were imaged with a pixel resolution of 63.1 nm (Zeiss Xradia Ultra 810), 0.36 μm (Zeiss Xradia Versa 520) and 7.92 μm (Zeiss Xradia Versa 520). The X-ray CT system (Zeiss Xradia Ultra 810) which achieved a resolution of 63.1 nm uses a chromium target with an accelerating voltage of 35 kV and tube current of 25 mA. The characteristic spectrum from the Cr target is quasi-monochromatic around 5.4 keV. The CT system used for the remaining scans (Zeiss Xradia Versa 520) had a characteristic spectrum from a tungsten target; the accelerating voltage and tube current are user defined and determine the peak intensity of the bremsstrahlung and photon flux. The accelerating voltage and tube current were chosen based on the X-ray absorption coefficients of the samples. The transmission images from all scans were reconstructed using a commercial software package (Zeiss XMReconstructor), which uses an algorithm based on standard filtered back-projection.Data processing
The reconstructed tomograms were processed using Avizo Fire 9 software (FEI VSG, France). An edge preserving non-local means filter31 was applied to the images to reduce noise while maintaining phase boundaries for segmentation. Phases were separated based on grey scale values, where the highly attenuating materials are displayed as white and weakly attenuating materials appear as dark grey. Measurements of sample porosity and particle size distribution were performed using Avizo Fire 9's label analysis tool. The temperature data and thermal imaging videos were extracted using FLIR's Altair software.Results and discussion
Voltage, temperature and operando tomography
During overcharge of lithium-ion batteries, a sequence of events related to the evolution of voltage, temperature and chemistry of the cell occurs leading up to thermal runaway and failure. In Fig. 1b, distinctive features can be identified in the temperature and voltage curves. For the initial 110 s (Region 1), the external temperature of the cell increases from 20 °C to ca. 40 °C. This initial heating is expected to derive primarily from irreversible heat generation mechanisms, such as Ohmic losses, which are most prevalent at high C rates.32 A plateau in cell voltage is observed after 110 s (Region 2 in Fig. 1b), followed by a significant increase in the rate of temperature rise after 120 s. The simultaneous voltage plateau and temperature rise indicates the initiation and progression of the decomposition/formation of the solid electrolyte interphase (SEI).33,34 At high temperatures (>80 °C), the SEI becomes unstable and self-discharge initiates. Lee et al.35 suggested that the lithium becomes more active within the carbon lattice at temperatures above 80 °C, causing a large reduction in charge transfer resistance. The combination of the accelerated loss of lithium and the instability of the SEI layer results in an increasing rate of the exothermic SEI decomposition/formation reactions and consumption of lithium.After ca. 80 s, corresponding to an external temperature of 40 °C, the pouch of the cell has begun to swell (Fig. 2) as gas is generated from reduction of the electrolyte and decomposition of the SEI.36–38 After 120 s, severe delamination is observed around the separator regions in the outer layers. Within Region 3 in Fig. 1b, a distinctive peak in cell voltage occurs over the space of ca. 40 s, consistent with the behaviour observed by Belov and Yang.39 In Movie S1 (ESI†), the pouch structure is seen to diverge from its original packaged structure, which corresponds with this voltage peak, as observed in Fig. 1b. It is expected that the rise in voltage is caused by the resistance increase associated with the gas pockets forming between the active layers, and the subsequent fall in voltage by the decrease in resistance when the pouch bursts and the gases are channelled away. The tightly wound active layers channel gases out into the exterior pouch when the pouch begins to lose its original package structure and starts to swell.
Finally, within Region 4 in Fig. 1b, the cell voltage rises sharply to the maximum voltage of the potentiostat, the most likely cause of which is the shutdown of the separator and damage to the internal circuit caused by the rupture of the cell at that time. The tomogram taken at 160 s in Fig. 2 shows delamination of the LiCoO2 layers from the aluminium current collector in various places, which may be evidence of the electrolyte vaporising or initial stages of decomposition of the LiCoO2 resulting in gas pockets forming between the aluminium current collector and the LiCoO2 layer. This may also be due to the current collector being oxidised by the electrolyte, or the products of electrolyte decomposition.40,41
The thermal decomposition of Li0.5CoO2 can occur at temperatures above 200 °C13 and the reaction between Li0.5CoO2 and solvent (ethylene carbonate), to produce CO2 and H2O, can occur at temperatures as low as 130 °C,10,13 according to eqn (1).
| Li0.5CoO2 + 0.1C3H4O3 → 0.5LiCoO2 + 0.5CoO + 0.3CO2 + 0.2H2O | (1) |
Fluctuations in the surface temperature of the cell occur after around 160 s as the cell ruptures (Movie S1, ESI†), at which point the unstable electrodes are exposed to air and the process of thermal runaway is accelerated. The heat generated from the reaction between the LiCoO2 and electrolyte, along with the decomposition of the SEI layer provide heat for the occurrence of thermal runaway which soon ensues, following which the surface temperature of the cell exceeds the limit of the thermal camera's calibrated range (Region 5 in Fig. 1b).
Post-mortem CT and battery architecture
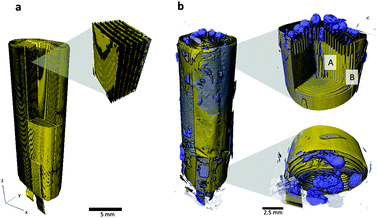
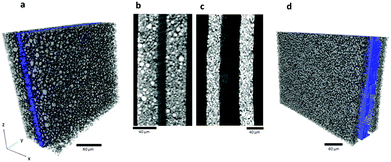
For comparison, the full cell was imaged in its fresh state (Fig. 3a) and also post-mortem (Fig. 3b), with a pixel size of 7.92 μm. The structure of the complete cell before and after thermal runaway is compared and the extent of structural damage and material degradation investigated. The aluminium (Al) phase was segmented (blue phase in Fig. 3b), and is shown to have agglomerated at the top and bottom of the wound electrode layers. The rapid gas generation from the decomposition of the LiCoO2 and electrolyte is likely to have carried the molten Al to the ends of the roll as the gas was channeled from the inner layers to the outer pouch. The porous structure of the Al globules at either end of the cell supports the hypothesis that gas played a part in carrying the molten metal. The uneven gas generation may also have had a peristaltic effect between the tightly wound layers, forcing the molten Al to either end.Fig. 4a–d compares the structure of the LiCoO2 electrode before and after thermal runaway. The pixel sizes for the fresh and post-mortem tomograms in Fig. 4 were 0.424 μm and 0.36 μm respectively. In its fresh state the LiCoO2 is coated uniformly onto the Al current collector (Fig. 4a and b) which is ca. 13 μm thick. As seen in Fig. 4c and d, the Al layer between the remains of the LiCoO2 is highly porous following thermal runaway and cooling. The LiCoO2 layers are much further apart, now ca. 40–50 μm, which would have aided the escape of gases generated during the thermal runaway process. The high specific heat and thermal conductivity of Al would also have enhanced the heat dissipation of local exothermic reactions leading up to and during thermal runaway. The melting point of Al is 660 °C and hence the distribution of molten aluminium remnants in Fig. 3 and 4 demonstrates that the interior of the cell reached in excess of 660 °C. As seen at the bottom of the cell in Fig. 3b, only a small portion of the cell in the region of the current collecting tab appears to have reached the melting point of copper (1085 °C). The temperature range between 660 °C and 1085 °C presents the possibility of further decomposition reactions occurring, evidence of which is found upon further investigation of the individual LiCoO2 particles post-mortem.
Micro-CT and particle structure degradation
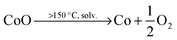
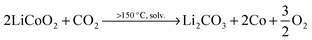
The size and shape of particles have been shown to play a significant role in the thermal stability of electrode materials and influence the magnitude of catastrophic failure upon thermal runaway.16,17 The decomposition reactions of LiCoO2 to Co3O4, CoO and further to Co metal occur at elevated temperatures and in the presence of organic electrolyte at the surface of the particles. For example, at >150 °C, the following reduction reactions can occur where solv. indicates the reaction step occurs in the presence of electrolyte solvent.10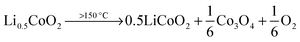 | (2) |
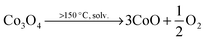 | (3) |
 | (4) |
 | (5) |
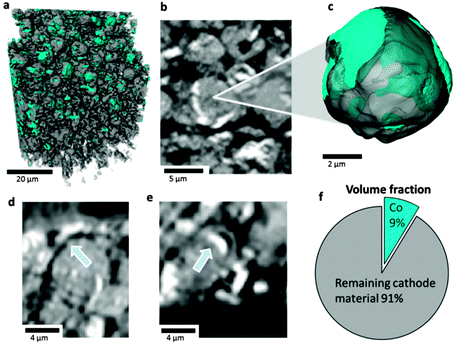 | ||
| Fig. 5 (a) 3D rendering of a portion of LiCoO2 electrode showing isolated Co phase (teal); (b) greyscale slice view of LiCoO2 particle showing evidence of phase separation based on attenuation and (c) semi-transparent 3D visualisation of LiCoO2 particle (grey) showing surface and sub-surface presence of Co (teal); (d and e) greyscale slice views showing delamination of Co metal surface layer; (f) 2D representation of the volume fraction of Co within the remaining LiCoO2 electrode material. This sample was extracted from an inner region labelled as A in Fig. 3. | ||
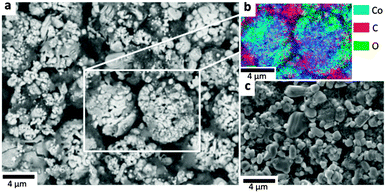
From attenuation coefficients of expected decomposition products (see ESI†) the composition of the particle surface layer and interior can be inferred; the difference in greyscale between the Co surface layer and the particle interior suggests that most of the particle consists of CoO, which is consistent with previous XRD experiments.10 The volume fraction of Co metal in the remaining cathode material is calculated to be 9% (Fig. 5f). SEM images and EDS (Fig. 6a and b) show that the surface of the particle consists of Co globules rather than the smooth surface presented in Fig. 5; this is due to the limited resolution of the X-ray CT technique to resolve fine surface features.26 The surface of the LiCoO2 particles after thermal runaway appears relatively rough when compared to the fresh sample presented in Fig. 6c, which is ascribed to shrinkage of the Co-oxide as it is reduced, and delamination and agglomeration of Co metal. Additionally, subsurface Co metal seams are present in the tomograms which could also be explained by the infiltration of electrolyte through micro-cracks and fractures, which could not be resolved in the CT images.
Without excess electrolyte and high temperature (>350 °C) it is expected that the air-stable Co3O4 and CoO would not be reduced to Co.43 Three samples were taken from subsurface layers (Region A in Fig. 3b), each of which showed the presence of Co on the surface when analysed under CT; however, a sample taken from the surface of the cell (region B in Fig. 3b) did not show any presence of Co on the surface of the particles (Fig. 7). This may have been due to the outer regions having a lower local temperature (due to enhanced heat rejection) and insufficient quantities of electrolyte for the final reduction step to occur. As shown in a previous study,19 temperature differences of >700 °C can occur over as little as 7 mm during thermal runaway, where the outer regions of the cell remain relatively cool and the inner regions can reach >1000 °C. Hence, at the outer regions the conditions (temperature and retention of electrolyte) are less favourable to sustaining the propagation of thermal runaway, making the extent of exothermic degradation location dependent.
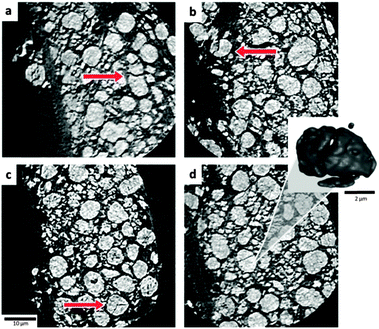 | ||
| Fig. 7 (a–d) Orthoslices highlighting cracked LiCoO2 particles. Sample was extracted from the outer region labelled as B in Fig. 3. Inset: An isolated 3D volume of the cracked particle in (d). | ||
In contrast to the subsurface samples, the surface sample presented in Fig. 7 contains numerous fractured particles. As Geder et al.17 demonstrated, a reduced particle size (and increased specific surface area) results in unfavourable consequences, such as a higher rate of heat generation and lower onset temperatures for thermal runaway.
Particle size distribution
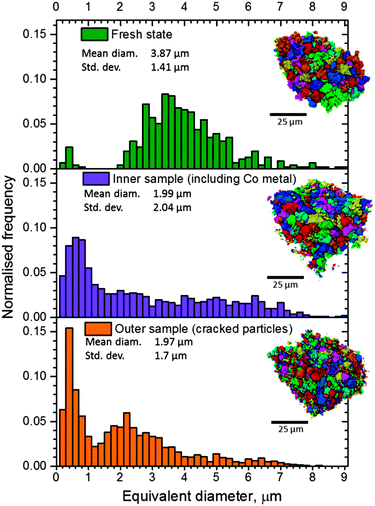
Mass loss occurs at each reduction step according to eqn (2)–(4), and the electrode particles are expected to reduce in mass and volume, and increase in density. The particle size distribution (PSD) of a fresh sample is compared with that of both post-mortem samples; the inner sample (showing presence of a Co surface layer) and the outer sample (showing significant cracking and the absence of Co). The particle volumes used for the PSD of the three samples were 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 μm3 (fresh), 34
897 μm3 (fresh), 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907 μm3 (inner), and 33
907 μm3 (inner), and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785 μm3 (outer). The PSD for each sample is presented in Fig. 8. In its fresh state the PSD centres around a mean diameter 3.87 μm with a relatively low standard deviation. There is a significant decrease in the mean diameter of the remaining particles for both post-mortem samples to approximately half their original size (to 1.99 μm and 1.97 μm for the inner and outer samples respectively), but the spread of data in the PSD greatly differs (Fig. 8). The PSD for the inner sample shows a single peak which has shifted below 2 μm, which is due to a combination of particle shrinkage during phase transitions and the presence of significant debris consisting of fractured particles and delaminated Co. In contrast, a bimodal PSD is observed for the outer sample (cracked particles). When compared to the PSD of the fresh sample, the twin peaks correspond to the occurrence of particle cracking, where the majority of the distribution is shifted to a smaller equivalent particle diameter. However, a second peak which consists of particles <1 μm in diameter contains the highest frequency of particles. This second peak may reflect smaller particle fragments from the fractured larger particles. Due to the limited resolution of the X-ray CT technique, the specific surface area of the particles could not be accurately determined; however, the dramatic reduction in particle size observed between the fresh and outer samples is expected to significantly affect the rate of decomposition of the electrode at elevated temperatures. Additional surface area would be exposed to reaction with the electrolyte and the smaller fragmented particles would exhibit a lower onset temperature.16,17 For example, as demonstrated by Jiang and Dahn,16 a reduction in particle size of LiCoO2 from 5 μm to 0.8 μm results in decreased thermal stability, with lower onset temperatures.
785 μm3 (outer). The PSD for each sample is presented in Fig. 8. In its fresh state the PSD centres around a mean diameter 3.87 μm with a relatively low standard deviation. There is a significant decrease in the mean diameter of the remaining particles for both post-mortem samples to approximately half their original size (to 1.99 μm and 1.97 μm for the inner and outer samples respectively), but the spread of data in the PSD greatly differs (Fig. 8). The PSD for the inner sample shows a single peak which has shifted below 2 μm, which is due to a combination of particle shrinkage during phase transitions and the presence of significant debris consisting of fractured particles and delaminated Co. In contrast, a bimodal PSD is observed for the outer sample (cracked particles). When compared to the PSD of the fresh sample, the twin peaks correspond to the occurrence of particle cracking, where the majority of the distribution is shifted to a smaller equivalent particle diameter. However, a second peak which consists of particles <1 μm in diameter contains the highest frequency of particles. This second peak may reflect smaller particle fragments from the fractured larger particles. Due to the limited resolution of the X-ray CT technique, the specific surface area of the particles could not be accurately determined; however, the dramatic reduction in particle size observed between the fresh and outer samples is expected to significantly affect the rate of decomposition of the electrode at elevated temperatures. Additional surface area would be exposed to reaction with the electrolyte and the smaller fragmented particles would exhibit a lower onset temperature.16,17 For example, as demonstrated by Jiang and Dahn,16 a reduction in particle size of LiCoO2 from 5 μm to 0.8 μm results in decreased thermal stability, with lower onset temperatures.
Although it is well known that the particle shape and size significantly affect the onset temperature and rate of heat generation during thermal runaway, it is not well understood how the evolving microstructure and likelihood of particle fracture influence these properties leading up to and during the runaway process. Further work is required to quantify the change in particle structure based on the conditions to which the particles are subjected, and the effect of initial particle morphology on the rate and quantity of heat generation during thermal runaway. As X-ray CT techniques continue to achieve higher spatial and temporal resolution, the dependence of morphological evolution on particle size, and its consequent effect on the magnitude of failure, could also be explored via operando micro-CT. A large particle size with low surface area may provide a more thermally stable electrode, but can also result in a reduction of the cell's power density; hence a balance between battery performance and safety may exist. Understanding the mechanisms through which particle architecture and properties change during thermal runaway is also essential to progress towards an optimised particle size distribution for both performance and safety.
Conclusions
This comprehensive approach of combined high speed operando X-ray CT and thermal imaging, followed by post-mortem multi-scale X-ray CT and SEM analyses, has provided new insights into the failure mechanisms of lithium-ion batteries. The effects of heating and gas generation on the architecture of the cell have been explored during failure via high-speed operando tomography, showing electrode layer delamination, gas pocket formation and consequent swelling of the pouch. Multi-scale comparative analyses of the complete cell, as well as separate components of the cell, before and after thermal runaway, have elucidated key phenomena that contribute to the propagation and severity of thermal runaway; the presence of agglomerated aluminium at both ends of the cell demonstrates the effective heat transfer throughout the cell via molten Al during failure.X-ray CT of the electrode particle microstructures in samples taken from different locations within the cell presents valuable information on the local kinetics and progress of exothermic reduction reactions at the positive LiCoO2 electrode. The volume fraction of Co, and its presence on the surface of the electrode particles, indicates that the rate of exothermic reduction steps, and therefore heat generation, is dependent on the surface area and PSD of the positive electrode. The extreme thermal and electrical conditions during overcharge-induced thermal runaway cause the particle morphology, and hence the PSD and specific surface area, to change.
The results of this work show that particle microstructure can significantly influence the extent and rate of exothermic degradation reactions during thermal runaway, and demonstrate that X-ray CT could be used to elucidate the relationship between electrode microstructure and overall safety of commercial cells.
Acknowledgements
The authors gratefully acknowledge funding from the Engineering and Physical Sciences Research Council (EPSRC), the Royal Academy of Engineering and the National Physical Laboratory (NPL). We are grateful to the STFC for travel funding through the Global Challenge Network in Batteries and Electrochemical Energy Devices. These experiments were performed on the ID15A beamline at the ESRF, Grenoble, France. The French National Research Agency (ANR) is acknowledged for support via EQUIPEX grant ANR-11-EQPX-0031 (project NanoimagesX).References
- NTSB, Aircraft Incident Report, Auxiliary Power Unit Battery Fire, Japan Airlines Boeing 787-8, JA829J, Report PB2014-108867, National Transport Safety Board, 2014.
- JTSB, Aircraft Serious Incident Investigation Report, All Nippon Airways Co., LTD JA804A, Report AI2014-4, Japan Transport Safety Board, 2014.
- AAIB, Report on the serious incident to Boeing B787-8, ET-AOP London Heathrow Airport on 12 July 2013, Report Air accident report: 2/2015, 2015.
- N.-S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y.-K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2009, 22, 587–603 CrossRef.
- UN Manual of Tests and Criteria, Part III: those relating to assignment of substances or articles to Class 2, Class 3, Class 4, Division 5.1, Class 8 or Class 9, United Nations, New York, 2009, Section 38.3: Lithium Metal and Lithium Ion Batteries.
- D. H. Doughty and C. Crafts, FreedomCAR Electrical Energy Storage System Abuse Test Manual for Electric and Hybrid Electric Vehicle Applications, Sandia National Laboratories, 2006 Search PubMed.
- F. Larsson and B.-E. Mellander, J. Electrochem. Soc., 2014, 161, A1611–A1617 CrossRef CAS.
- C.-H. Doh, D.-H. Kim, H.-S. Kim, H.-M. Shin, Y.-D. Jeong, S.-I. Moon, B.-S. Jin, S. W. Eom, H.-S. Kim, K.-W. Kim, D.-H. Oh and A. Veluchamy, J. Power Sources, 2008, 175, 881–885 CrossRef CAS.
- D. D. MacNeil and J. R. Dahn, J. Electrochem. Soc., 2002, 149, A912–A919 CrossRef CAS.
- R. A. Leising, M. J. Palazzo, E. S. Takeuchi and K. J. Takeuchi, J. Power Sources, 2001, 97–98, 681–683 CrossRef CAS.
- R. A. Leising, M. J. Palazzo, E. S. Takeuchi and K. J. Takeuchi, J. Electrochem. Soc., 2001, 148, A838–A844 CrossRef CAS.
- D. D. MacNeil and J. R. Dahn, J. Electrochem. Soc., 2001, 148, A1205–A1210 CrossRef CAS.
- D.-W. Chung, P. R. Shearing, N. P. Brandon, S. J. Harris and R. E. García, J. Electrochem. Soc., 2014, 161, A422–A430 CrossRef CAS.
- M. Jo, Y.-S. Hong, J. Choo and J. Cho, J. Electrochem. Soc., 2009, 156, A430–A434 CrossRef CAS.
- J. Jiang and J. R. Dahn, Electrochim. Acta, 2004, 49, 2661–2666 CrossRef CAS.
- J. Geder, H. E. Hoster, A. Jossen, J. Garche and D. Y. W. Yu, J. Power Sources, 2014, 257, 286–292 CrossRef CAS.
- J. R. Dahn, E. W. Fuller, M. Obrovac and U. von Sacken, Solid State Ionics, 1994, 69, 265–270 CrossRef CAS.
- D. P. Finegan, M. Scheel, J. B. Robinson, B. Tjaden, I. Hunt, T. J. Mason, J. Millichamp, M. Di Michiel, G. J. Offer, G. Hinds, D. J. L. Brett and P. R. Shearing, Nat. Commun., 2015, 6, 6924 CrossRef CAS PubMed.
- P. G. Balakrishnan, R. Ramesh and T. Prem Kumar, J. Power Sources, 2006, 155, 401–414 CrossRef CAS.
- Z. Chen, Y. Qin and K. Amine, Electrochim. Acta, 2009, 54, 5605–5613 CrossRef CAS.
- C. F. Lopez, J. A. Jeevarajan and P. P. Mukherjee, J. Electrochem. Soc., 2015, 162, A1905–A1915 CrossRef CAS.
- M. Ebner, F. Marone, M. Stampanoni and V. Wood, Science, 2013, 342, 716–720 CrossRef CAS PubMed.
- D. P. Finegan, E. Tudisco, M. Scheel, J. B. Robinson, O. O. Taiwo, D. S. Eastwood, P. D. Lee, M. Di Michiel, B. Bay, S. A. Hall, G. Hinds, D. J. L. Brett and P. R. Shearing, Adv. Sci., 2016, 3, 1500332, DOI:10.1002/advs.201500332.
- D. S. Eastwood, V. Yufit, J. Gelb, A. Gu, R. S. Bradley, S. J. Harris, D. J. L. Brett, N. P. Brandon, P. D. Lee, P. J. Withers and P. R. Shearing, Adv. Energy Mater., 2014, 4, 1300506 Search PubMed.
- P. R. Shearing, N. P. Brandon, J. Gelb, R. Bradley, P. J. Withers, A. J. Marquis, S. Cooper and S. J. Harris, J. Electrochem. Soc., 2012, 159, A1023–A1027 CrossRef CAS.
- V. Yufit, P. Shearing, R. W. Hamilton, P. D. Lee, M. Wu and N. P. Brandon, Electrochem. Commun., 2011, 13, 608–610 CrossRef CAS.
- A. Rack, M. Scheel, L. Hardy, C. Curfs, A. Bonnin and H. Reichert, J. Synchrotron Radiat., 2014, 21, 815–818 CAS.
- M. Di Michiel, J. M. Merino, D. Fernandez-Carreiras, T. Buslaps, V. Honkimäki, P. Falus, T. Martins and O. Svensson, Rev. Sci. Instrum., 2005, 76, 043702 CrossRef.
- A. Kyrieleis, M. Ibison, V. Titarenko and P. J. Withers, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 607, 677–684 CrossRef CAS.
- A. Buades, B. Coll and J. Morel, Multiscale Model. Simul., 2005, 4, 490–530 Search PubMed.
- C. Heubner, M. Schneider and A. Michaelis, J. Power Sources, 2016, 307, 199–207 CrossRef CAS.
- Z. Chen, Y. Qin, Y. Ren, W. Lu, C. Orendorff, E. P. Roth and K. Amine, Energy Environ. Sci., 2011, 4, 4023–4030 CAS.
- H. Yang, H. Bang, K. Amine and J. Prakash, J. Electrochem. Soc., 2005, 152, A73–A79 CrossRef CAS.
- H. H. Lee, C. C. Wan and Y. Y. Wang, J. Electrochem. Soc., 2004, 151, A542–A547 CrossRef CAS.
- T. Ohsaki, T. Kishi, T. Kuboki, N. Takami, N. Shimura, Y. Sato, M. Sekino and A. Satoh, J. Power Sources, 2005, 146, 97–100 CrossRef CAS.
- A. W. Golubkov, D. Fuchs, J. Wagner, H. Wiltsche, C. Stangl, G. Fauler, G. Voitic, A. Thaler and V. Hacker, RSC Adv., 2014, 4, 3633–3642 RSC.
- J.-H. Kim, N. P. W. Pieczonka and L. Yang, ChemPhysChem, 2014, 15, 1940–1954 CrossRef CAS PubMed.
- D. Belov and M.-H. Yang, ECS Trans., 2007, 6, 29–44 CAS.
- E. Krämer, S. Passerini and M. Winter, ECS Electrochem. Lett., 2012, 1, C9–C11 CrossRef.
- D. Belov and M.-H. Yang, J. Solid State Electrochem., 2008, 12, 885–894 CrossRef CAS.
- C.-K. Lin, Y. Ren, K. Amine, Y. Qin and Z. Chen, J. Power Sources, 2013, 230, 32–37 CrossRef CAS.
- Z. Lendzion-Bieluń, R. Jędrzejewski and W. Arabczyk, Cent. Eur. J. Chem., 2011, 9, 834–839 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp04251a |
| This journal is © the Owner Societies 2016 |