Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stability of LAPONITE®-stabilized high internal phase Pickering emulsions under shear†
M.
Dinkgreve
*a,
K. P.
Velikov
bc and
D.
Bonn
a
aVan der Waals-Zeeman Institute, Institute of Physics, University of Amsterdam, Science Park 904, 1018 XH Amsterdam, The Netherlands. E-mail: m.dinkgreve@uva.nl
bUnilever R&D Vlaardingen, Olivier van Noortlaan 120, 3133 AT Vlaardingen, The Netherlands
cSoft Condensed Matter, Debye Institute for Nanomaterials Science, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands
First published on 27th July 2016
Abstract
Colloidal particles are often used to make Pickering emulsions that are reported to be very stable. Commonly the stabilization is a combined effect of particle adsorbing at the fluid interface and a particle network in the continuous phase; the contribution of each to the overall stability is difficult to assess. We investigate the role of LAPONITE® particles on high internal phase emulsion stability by considering three different situations: emulsion stabilization by surfactant only, by surfactant plus clay particles, and finally clay particles only. To clarify the structure of the emulsion and the role of the clay particles, we have succeeded in fluorescently labelling the clay particles by adsorbing the dye onto the particle surfaces. This allows us to show directly using confocal microscopy, that the clay particles are not only located at the interface but also aggregate and form a gel in the continuous aqueous phase. We show that the emulsions in the presence of surfactant (with or without clay) are stable to coalescence and shear. Without surfactant (with only LAPONITE® as stabilizer) the emulsions are stable to coalescence for several weeks, however they destabilize rapidly under shear. Our results suggest that the formation of the emulsions is mostly due to gel formation of the clay particles in the continuous phase, rather than that the clay is an emulsifier. This gel formation also accounts for the instability of the emulsions to shear that we observe caused by shear thinning of the continuous gel and inability of the adsorbed particles to rearrange effectively around the droplets due to their attractive nature.
I. Introduction
Emulsions are of great importance for the cosmetic, oil and food industry. Most frequently, emulsions are stabilized with surfactants,1 however these are not always stable under shear2 and alternative stabilization mechanisms have therefore been intensively explored in recent years.3 Amongst these, particle stabilized emulsions have attracted much attention, notably for their high stability and possible role in food applications, in the design of more environmentally friendly agricultural formulations and for the realization of high internal phase emulsions.4 This type of emulsion is called Pickering emulsion; particles are adsorbed at the oil–water interface with a high stabilization energy and are believed to form a continuous layer around the dispersed drops impeding coalescence5 and hence stabilizing the emulsion. Such Pickering emulsions are stabilized by solid particles only.6–9 If a surfactant is also present, the particle wetting preference can be changed and possibly the particles will not adsorb. In this case, another type of emulsion can form that is mainly stabilized by the presence of a surfactant layer at the surface of the drops. The colloidal particles in these emulsions have been shown to induce a depletion attraction between the emulsion droplets that can cause these emulsions to be thixotropic, i.e., have a reversible time-dependent rheology.10 Moreover, aging effects are observed in surfactant-stabilised bubbles dispersed within LAPONITE® gels.11Clays are one of the most-used solid particles in emulsion stabilization, often with rather high clay concentrations. These high concentrations make it likely that the continuous aqueous phase between the oily emulsion droplets forms a gel, which should influence both the stability and the rheology of the emulsions.12–14 Such an effect does not appear to have been taken into account in prior experiments on emulsion stability and rheology in the presence of clay particles.15–18 Reports that do take the gelation of clay particles into account are always in the presence of a surfactant. Lagaly et al.19 discussed the two mechanisms by which clay in addition to a surfactant based emulsion can stabilize drops. The first is “stabilization by envelopes of particles around the oil droplets” and the second is “stabilization by entrapment and immobilization of oil drops in three-dimensional network of particles”. They also remark that the stabilization by a particle network may be comparable to the role of liquid-crystalline phases in stabilizing emulsions.20 Bon and Clover21 used LAPONITE® as stabilizer in the miniemulsion polymerization of styrene to obtain latex particles armored with LAPONITE® particles. They showed that a three-dimensional network of clay particles in the continuous phase of the emulsions is required to obtain a stable LAPONITE® coated latex. All these observations pose the question what the difference is between clay and clay/surfactant stabilized emulsions. This question is particularly important for concentration emulsions where shear induced instabilities have a higher impact on droplet coalescence and hence emulsion stability.
In this article we compare the stability to shear of three emulsion systems; the first is stabilized with surfactant only, the second with surfactant and clay particles and finally emulsions stabilized with clay particles only. The emulsions themselves are castor oil-in-water and silicone oil-in-water emulsions. The three different systems have different continuous phases: surfactant only, surfactant + clay, and clay only. The surfactant used is Sodium Dodecyl Sulfate (SDS, from Sigma Aldrich), that is dissolved in ultra-pure water (Milli-Q®) obtaining a solution of 1 wt% (∼35 mM) SDS concentration. To study the influence of clay, different concentration of LAPONITE® RD are added before emulsifying (in wt% of the total weight of the emulsion). Here we consider rather high clay concentration and high surfactant (ionic strength) or salt concentrations, therefore an attractive gel forms.22 LAPONITE® is a synthetic clay that consists of disc-like particles, with a diameter of 30 nm and thickness of 1 nm obtained from BYK Additives & Instruments.23 The LAPONITE® disks have a negative charge on the sides and a slightly positive charged on the edges. For the emulsions stabilized with clay particles only, similar as in previous works,15 the continuous aqueous phase is first prepared by suspending 2 wt% LAPONITE® RD in a 0.1 M Sodium Chloride (NaCl, Ultrapure, from Sigma Aldrich) solution in ultra-pure water (Millipore Milli-Q). To disperse the particles, we use a high intensity ultrasonic disperser (Branson solifier 250, tip diameter 5 mm), operating at 20 kHz and 100 W for 30 minutes.
For optical microscopy observations, we prepare transparent emulsions in which the continuous phase is refractive index matched to the oil phase (Silicone oil Rhodorsil® 47 V 500) by adding glycerol (99% GC, from Sigma-Aldrich) to the aqueous phase.
For all emulsions, the oil is gradually added to the aqueous phase when stirring with a Silverson L5M mixer at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 minutes. Emulsions with different volume fractions ϕ are prepared by diluting the original emulsion (ϕoil = 0.70) with the corresponding continuous phase. We study the structure and stability of these emulsions by direct visual observation and confocal fluorescence microscopy (Zeiss LSM Live). The rheology of these emulsions was studied on an Anton Paar MCR302 rheometer equipped with a cone plate geometry of 50 mm radius and with a cone angle of 1° with roughened surfaces to avoid wall slip.24 To prevent evaporation of the sample a vapor trap is used during all the rheological tests.
000 rpm for 2 minutes. Emulsions with different volume fractions ϕ are prepared by diluting the original emulsion (ϕoil = 0.70) with the corresponding continuous phase. We study the structure and stability of these emulsions by direct visual observation and confocal fluorescence microscopy (Zeiss LSM Live). The rheology of these emulsions was studied on an Anton Paar MCR302 rheometer equipped with a cone plate geometry of 50 mm radius and with a cone angle of 1° with roughened surfaces to avoid wall slip.24 To prevent evaporation of the sample a vapor trap is used during all the rheological tests.
Emulsions with surfactant
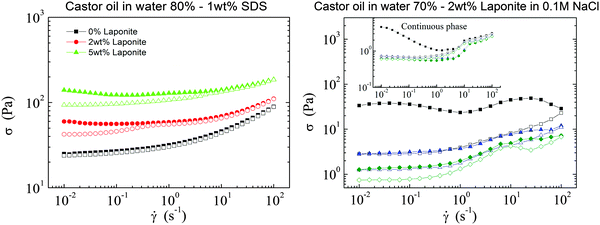
The emulsions stabilized with SDS are stable for months to coalescence and are stable to shear. Fig. 1a shows an up-and-down shear rate sweep for castor oil-in-water emulsion (ϕoil = 0.80) with SDS and different concentrations of LAPONITE®. We observe that without clay, the flow curve is perfectly reversible doing an up-and-down shear rate sweep. However, by adding clay to the emulsion, the flow curves develop a hysteresis that becomes more pronounced with increasing clay content. These emulsions can be characterized rheologically as a thixotropic yield stress material.10 Besides the thixotropy, the addition of clay does not have a significant effect on the emulsions stability in these experiments. By the definition of thixotropy, the changes in the rheological behavior are reversible; they are also reversible in these rheology experiments, showing that the shear induced no macroscopic destabilization of the emulsion itself.Emulsions with clay only
If emulsions are only stabilized with clay, we find that the emulsions exhibit good stability to coalescence at rest; leaving the samples for several weeks does not result in any visible changes of the aspect of the emulsion. However, during the rheological measurements the emulsions destabilize quite spectacularly. Fig. 1b shows the result of up-and-down shear rate sweeps performed repeatedly (three times consecutively) on the castor oil-in-water emulsion. Similar results are obtained for the silicone oil-in-water emulsion, see ESI.† The emulsions show a rapid decrease in viscosity when performing multiple shear rate sweeps. This is in stark contrast to what is observed for surfactant-stabilized emulsions, for which the flow curve is perfectly reversible in up-and-down stress.Visual inspection after shearing reveals that the LAPONITE® stabilized emulsions are unstable to shear; after the rheological tests a clear phase separation between the oil and the water phases is observed. A similar decrease of the viscosity is observed if the same experiment is done on the continuous phase only. At the clay and salt concentrations that compose the continuous phase, aqueous LAPONITE® suspensions are known to form an attractive colloidal gel that is very sensitive to shear.25 The rheological experiment shows that after the first up-and-down shear rate sweep, the viscosity decreases by an order of magnitude, and remains very low if the sample is sheared in subsequent sweeps (Fig. 1, inset). The picture that emerges from the ensemble of these experiments is that the flow destabilizes the colloidal clay gel that forms the continuous phase separating the droplets, allowing them to contact each other and coalesce, which in turn destabilizes the emulsion.
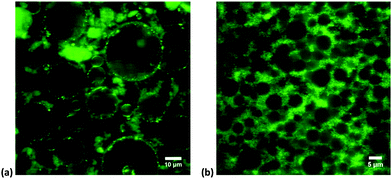
To check this scenario on a more microscopic level, confocal fluorescence microscopy is used to visualize both the LAPONITE® suspension by itself and the emulsions containing LAPONITE®. As a fluorescent dye we use Rhodamine 6G (from Sigma Aldrich), a hydrophilic fluorescent molecule that is completely adsorbed onto the LAPONITE® particles. The behavior of the transparent silicone oil-in-water emulsions, before, during and after shearing, is observed with the confocal microscope in combination with a rheometer.
The LAPONITE® particles are clearly visible; they form aggregated flocs in the continuous phase, but are also clearly visible on the interface of the oil droplets with the continuous phase (Fig. 2a). The continuous phase is more heterogeneous. In contrast, in the presence of surfactant the clay particles form a continuous (homogeneous) network surrounding the particles and are not specifically located at the interface (Fig. 2b). Moreover, the droplet size is different.
Separate observation on the aqueous LAPONITE® phase alone (before emulsification with the oil) also reveals the formation of a colloidal gel with a very similar structure. The continuous phase is a soft elastic solid that does not flow under the influence of gravity (Fig. 3). To asses the effect of flow, we shear for 30 seconds at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 100 s−1 and visualize the LAPONITE® structure before and after shear. We observe before shear a continuous network, whereas after shear the network breaks into smaller aggregates and does not appear to be percolated anymore. It is this gel that destabilizes under shear that leads to destabilization of the emulsion. In contrast, an emulsion with a surfactant does not destabilize.
= 100 s−1 and visualize the LAPONITE® structure before and after shear. We observe before shear a continuous network, whereas after shear the network breaks into smaller aggregates and does not appear to be percolated anymore. It is this gel that destabilizes under shear that leads to destabilization of the emulsion. In contrast, an emulsion with a surfactant does not destabilize.
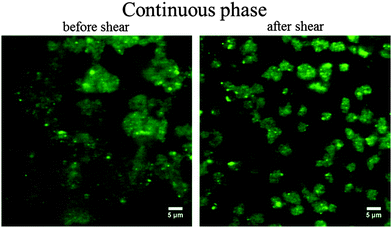 | ||
Fig. 3 Confocal fluorescence images of continuous phase; 2 wt% LAPONITE® in 0.1 M NaCl (LAPONITE® dyed with Rhodamine 6G). Before and after shear (shear for 30 seconds at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 100 s−1). = 100 s−1). | ||
In the usual definition of Pickering emulsions, the particles are localized at the interface to form a dense film that protects the droplets from coalescence. It is for this reason that such emulsions are believed to be very stable; for spherical particles the stabilization energy depends on the particle size6 but is typically of the order of a few hundred kBT. If the emulsions solely stabilized with LAPONITE® have a similar protective particle film on the drop surfaces, one would expect a similar stability for the LAPONITE® stabilized emulsions. LAPONITE® particles are not spherical but disk-like, but still if the particles are at the interface stabilization energies should be similar. We find however that although the emulsions are stable at rest, they are rapidly destabilized by shear, and hence the LAPONITE® layer at the surface does not provide a significant barrier against coalescence. The observation that the rheology of the emulsions and the continuous LAPONITE® phase are similar suggests that the stability of the emulsions at rest is due to the fact that the oil droplets are embedded in the visco-elastic LAPONITE® gel, that prevents the droplets from coalescencing. When the colloidal gel is destroyed by the shear allowing the droplets to move and get in contact, coalescence is rapid, in agreement with all of the observations above. The aggregated LAPONITE® particles on the surface are not able to rearrange to provide an effective barrier against coalescence, but rather form particle aggregates that do not provide an effective coverage. Garcia and Whitby also observe this behavior, but coalescence was not detected due to lower volume fraction of oil. Whitby and co-authors, however, observed the shear induced coalescence in oil-in-water Pickering emulsions stabilized by silanized fumed silica particles subjected to simple shear flow.25
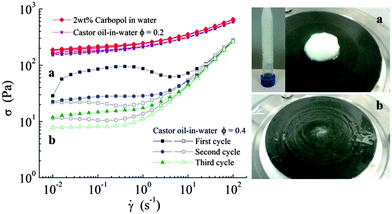
As an independent test of this idea, we can make emulsions without any particles or surfactants, but with the continuous phase being a gel with a yield stress.27 We therefore have prepared emulsions with a Carbopol gel as the continuous phase. Carbopol consists of polymer particles that swell tremendously in water with the right pH; our gels are prepared by mixing 2 wt% Carbopol (Ultrez U10 grade) and ultrapure water. A solution of 18 wt% Sodium Hydroxide (NaOH, from Sigma Aldrich) was dissolved in water and added to the Carbopol mixture to adjusted the pH to approximately 7.28 This gives a simple yield stress fluid,26 from which subsequently castor oil-in-water ‘emulsions’ (ϕ = 0.4; for higher concentrations of castor oil we observe phase inversion of the emulsion) are prepared using the same procedure as for the LAPONITE® emulsions. We find that the Carbopol ‘emulsions’ show a similar rheology as we observe for the LAPONITE®-stabilized emulsions (Fig. 4); a very stable ‘emulsion’ at rest that is rapidly destabilized by shearing. When the continuous phase is a yield stress fluid but nothing happens at the oil–water surface, emulsions can be stable at rest but not under shear. The similarity with the results for LAPONITE® strongly suggest that the mechanism is the same there.
II. Conclusions
We have investigated the stability of LAPONITE® stabilized high internal phase emulsions. A simple rheological test (steady shear) shows that the emulsions are not stable to shear and undergo a catastrophic coalescence. Therefore, our ‘Pickering’ emulsion, as evident from confocal microscopy, behaves merely as a dispersion of oil droplets in a LAPONITE® gel. It is this gel that destabilizes under shear that leads to destabilization of the emulsion, this follows from the observation that an emulsion with a good emulsifier (a surfactant) does not destabilize. This point is reinforced if we look at the conditions of formation of LAPONITE®-stabilized emulsions. Such emulsions were studied previously;29 but with lower salt concentrations and lower oil volume fractions that are also in a region of the LAPONITE®/salt phase diagram where the continuous phase becomes a soft visco-elastic solid. Binks and collaborators have shown that in their systems, LAPONITE® stabilized emulsions are only stable if enough salt is added to effectively screen the electrostatic interactions between the LAPONITE® particles and hence favor the formation of the colloidal gel. If our emulsions are made with a liquid suspension of the same concentration of LAPONITE® (for 2 wt% of LAPONITE®) but without salt, no stable emulsions are obtained. Thus we conclude that the colloidal gel formation is the essential ingredient for the emulsion preparation, however the extend of shear thinning and recovery speed of the colloidal gel play critical role in the emulsion stabilization.References
- J. Sjöblom, Emulsions and Emulsion Stability: Surfactant Science Series, CRC Press, Taylor & Francis Group, New York, 2006, vol. 132 Search PubMed.
- A. Deblais, R. Harich, D. Bonn, A. Colin and H. Kellay, Langmuir, 2015, 31.22, 5971–5981 CrossRef PubMed.
- C. C. Berton-Carabin and K. Schroën, Annu. Rev. Food Sci. Technol., 2005, 6, 263–297 CrossRef PubMed.
- G. Sun, Z. Li and T. Ngai, Angew. Chem., 2010, 49.12, 2163–2166 CrossRef PubMed.
- S. Levine, B. D. Bowen and S. J. Partridge, Colloids Surf., 1989, 38, 325–343 CrossRef CAS.
- S. U. Pickering, J. Chem. Soc., 1907, 91, 2001–2021 RSC.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CrossRef CAS.
- B. Liu, W. Wei, X. Qu and Z. Yang, Angew. Chem., 2008, 120, 4037–4039 CrossRef.
- S. Sacanna, W. K. Kegel and A. P. Philipse, Phys. Rev. Lett., 2009, 98, 158301 CrossRef PubMed.
- A. Ragouilliaux, G. Ovarlez, N. Shahidzadeh-Bonn, B. Herzhaft, T. Palermo and P. Coussot, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 76, 051408 CrossRef CAS PubMed.
- R. M. Guillermic, A. Salonen, J. Emile and A. Saint-Jalmes, Soft Matter, 2009, 5, 4975–4982 RSC.
- S. Jabbari-Farouji, H. Tanaka, G. H. Wegdam and D. Bonn, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 78, 061405 CrossRef CAS PubMed.
- M. Dijkstra, J. P. Hansen and P. A. Madden, Phys. Rev. Lett., 1995, 75, 2236 CrossRef CAS PubMed.
- B. Ruzicka, L. Zulian and G. Ruocco, Phys. Rev. Lett., 2004, 93, 258301 CrossRef CAS PubMed.
- N. P. Ashby and B. P. Binks, Phys. Chem. Chem. Phys., 2000, 2, 5640–5646 RSC.
- Y. Jung, Y. H. Son, J. K. Lee, T. X. Phuoc, Y. Soong and M. K. Chyu, ACS Appl. Mater. Interfaces, 2011, 3.9, 3515–3522 Search PubMed.
- C. Li, Q. Liu, Z. Mei, J. Wang, J. Xu and D. Sun, J. Colloid Interface Sci., 2009, 336, 314–321 CrossRef CAS PubMed.
- Y. Nonomura and N. Kobayashi, J. Colloid Interface Sci., 2009, 330, 463–466 CrossRef CAS PubMed.
- G. Lagaly, M. Reese and S. Abend, Appl. Clay Sci., 1999, 14, 83–103 CrossRef CAS.
- S. E. Friberg, L. B. Goldsmith and M. L. Hilton, Theory of emulsions, Pharmaceutical Dosage Forms: Disperse Systems, Marcel Dekker Inc., New York, 1988, pp. 49–91 Search PubMed.
- S. A. F. Bon and P. J. Colver, Langmuir, 2007, 23.16, 8316–8322 CrossRef PubMed.
- B. Ruzicka and E. Zaccarelli, Soft Matter, 2011, 7, 1268–1286 RSC.
- LAPONITE® RD is manufactured by BYK Additives & Instruments.
- A. Fall, J. Paredes and D. Bonn, Phys. Rev. Lett., 2010, 105, 225502 CrossRef PubMed.
- B. Abou, D. Bonn and J. Meunier, J. Rheol., 2003, 47, 979–988 CrossRef CAS.
- C. P. Whitby, F. E. Fischer, D. Fornasiero and J. Ralston, J. Colloid Interface Sci., 2011, 361.1, 170–177 CrossRef PubMed.
- M. M. Denn and D. Bonn, Rheol. Acta, 2011, 50.4, 307–315 CrossRef.
- P. C. M. Møller, A. Fall and D. Bonn, EPL, 2009, 87, 38004 CrossRef.
- P. C. Garcia and C. P. Whitby, Soft Matter, 2012, 8, 1609–1615 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp03572h |
| This journal is © the Owner Societies 2016 |