Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interaction of BODIPY dyes with bovine serum albumin: a case study on the aggregation of a click-BODIPY dye†
Laramie P.
Jameson
,
Nicholas W.
Smith
,
Onofrio
Annunziata
and
Sergei V.
Dzyuba
*
Department of Chemistry and Biochemistry, Texas Christian University, Fort Worth, TX 76129, USA. E-mail: s.dzyuba@tcu.edu
First published on 9th May 2016
Abstract
The fluorescence of BODIPY and click-BODIPY dyes was found to substantially increase in the presence of bovine serum albumin (BSA). BSA acted as a solubilizer for dye aggregates, in addition to being a conventional binding scaffold for the click-BODIPY dyes, indicating that disaggregation of fluorophores should be considered when evaluating dye–protein interactions.
BODIPY dyes are among the most useful and versatile small molecule fluorescent probes, and a wide range of applications has been attributed to the dyes' high thermal and chemical stabilities, high quantum yields, extinction coefficients, as well as tunable spectroscopic properties.1–3 In regard to biomolecular processes, BODIPY dyes have been primarily used to label ligands to address ligand–receptor interactions.4,5 Recently, several reports suggested that BODIPY dyes could interact directly with a variety of proteins and peptide assemblies, acting as fluorescence-based sensors.6,7
Fluorophore–albumin interactions are of interest, since serum albumins are the major small molecule-binding proteins, which are considered suitable models for various in vitro studies on ligand–protein interactions.8 In addition, due to its size and collection of binding sites, bovine serum albumin (BSA) could be viewed as a viable model for non-specific binding. Although a number of fluorophores have been shown to bind to albumins, only a few BODIPY dyes have been investigated.9–13 Notably, a BODIPY dye was identified (out of a library of 137 dyes) that exhibited ca. 200-fold emission enhancement in the presence of BSA, while exhibiting high specificity towards BSA over serum albumins from other species (human, porcine, rat, and sheep).11,12 Significantly, specifically substituted BODIPY-based fluorescent probes were shown to be viable sensors of protein hydrophobicity.14 Furthermore, several common BODIPY dyes, including a water-soluble derivative, were recently suggested to interact with albumins15 as was evidenced by an increase in the emission intensity.
We previously demonstrated that the incorporation of a triazole moiety on the BODIPY dye scaffold afforded probes that had a significant affinity towards soluble oligomers of amyloid peptides,16 thus illustrating the possibility for click-BODIPY dyes to act as biosensors.
Here, in order to expand on the utility of BODIPY dyes, we examined the interactions between triazole-containing BODIPY dyes, so-called click-BODIPY dyes, (Fig. 1) and BSA. The incorporation of the triazole group onto the BODIPY scaffold was accomplished in a straightforward manner using an alkyne-containing BODIPY scaffold (ESI†). Dye 2 has a triazole moiety, and the presence of the methyl group, rather than the benzyl group, assures that dye 2 is less hydrophobic than dye 3.
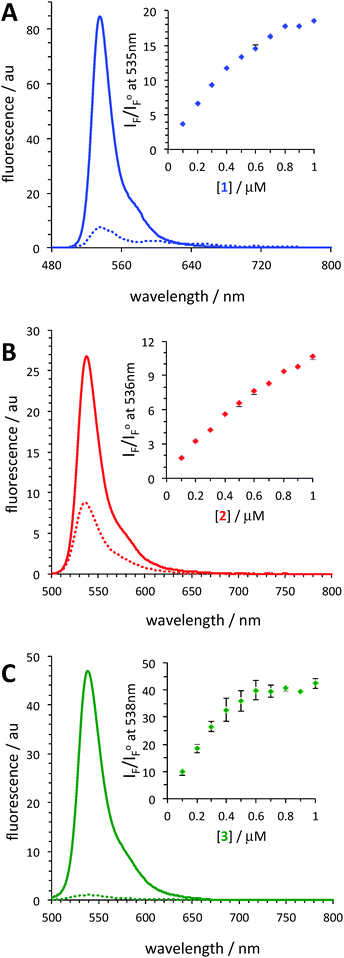
During the initial screening, the fluorescence of dyes 1, 2, and 3 was measured in the presence of a fixed amount of BSA (39.2 μM) and a notable enhancement in the fluorescence of the dyes in the presence of BSA was observed (Fig. 2).
At the highest experimental dye concentration (1 μM), krypto-BODIPY dye 1 exhibited a ca. 20-fold increase in its fluorescence intensity in the presence of BSA, while the introduction of the methyl-triazole moiety, dye 2, resulted in only a 10-fold increase. Remarkably, in the presence of BSA, dye 3 exhibited a significantly larger enhancement, ca. 40-fold. Notably, the fluorescence enhancement of dye 3 compared to that of dyes 1 and 2 could be viewed as even more remarkable at lower dye concentrations (e.g., 0.2 μM), since the fluorescence of dye 3 saturated at dye concentrations greater than 0.5 μM. It should also be pointed out that the absorption spectra of the dyes were not drastically different in the absence and presence of BSA (Fig. S1–S6, ESI†).
Furthermore, the fluorescence intensity of dye 3, in the presence of BSA, was found to increase with time, t, towards its asymptotic equilibrium value. This was likely related to the kinetics of protein–dye association and the corresponding desolvation effects of the dye and its aggregates. This behavior was not observed in the case of the other two dyes, i.e., no time-dependent increase in the emission was observed upon addition of dyes 1 and 2 to the solution of BSA. For dye 3, the fluorescence intensity at equilibrium, IF(∞) was obtained by fitting the time-dependent fluorescence, iF(t), to the first-order kinetic expression IF(∞)[1 − a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−bt)], where IF(∞) a, and b are fitting parameters. A representative profile is shown in Fig. 3.
exp(−bt)], where IF(∞) a, and b are fitting parameters. A representative profile is shown in Fig. 3.
The aforementioned observations may be related to the presence of the benzyl group on dye 3, which increases the hydrophobicity of the dye, and as such the dye's interaction with hydrophobic binding pockets of the protein should be favored. However, the saturation of the fluorescence signal was taking place at dye concentrations of ca. 0.5 μM (Fig. 2C). In order to gain insight into the BODIPY–BSA interactions, we carried out more detailed titration experiments at dye concentrations that were below the saturation point using fluorescence spectroscopy.
The titration conditions were chosen such that the total concentration of protein P (CP) was large enough when compared to the total concentration of the dye D (CD). Thus, it could be assumed that only a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of protein–dye (PD), would form appreciably, irrespective of the number of binding sites of BSA. This reversible interaction could be represented as P + D ⇌ PD, with the following mass action law (eqn (1)):
1 complex of protein–dye (PD), would form appreciably, irrespective of the number of binding sites of BSA. This reversible interaction could be represented as P + D ⇌ PD, with the following mass action law (eqn (1)):
 | (1) |
| F = 1 + (R − 1)α | (2) |
 | (3) |
 | ||
| Fig. 4 Experimental fluorescence ratios (F = IF/IoF) as a function of protein concentration, CP, at constant dye concentration, CD = 0.20 μM, for dyes 1 (blue), 2 (red), and 3 (green); λex = 530 nm; λem = 538 nm; buffer: 10 mM TRIS (0.1 M NaCl, pH 7.4); the data are the average of 2–3 measurements ± SD. The solid curves are the theoretical fits through the experimental data, obtained using eqn (4) and (5). | ||
The observed saturation in the fluorescence behavior of dye 3 (Fig. 2C) required additional explanation. At 0.5 μM of the dye, the protein was present in a large excess as compared to the dye, and the saturation of a binding site was unlikely. We considered the self-aggregation of dye 3 as a competitor to the protein–dye binding in solution. Furthermore, these aggregates were assumed to have a minor contribution to the overall fluorescence, since typically aggregation-induced quenching of fluorescence is reported for the vast majority of fluorophores.17,18
In regard to the dye–BSA interaction, the dye aggregation could be assessed when fluorescence intensity (expressed as IF − IoF, where IoF is the fluorescence intensity of the protein-free system) is plotted as a function of CD at constant CP (Fig. 4). At low protein concentrations, IF − IoF reached a plateau as the dye concentration was increased. This plateau decreased as the protein concentration increased. It is important to note that the protein concentration was significantly larger than that of the dye in all cases, which precludes the saturation of the protein's binding sites. In this case, the protein likely acted as a solubilizer for the dye, and thereby reduced the amount of dye aggregates in solution. Such solubilization by BSA could also explain the disappearance of the plateau in the titration experiments at higher protein concentrations (Fig. 5).
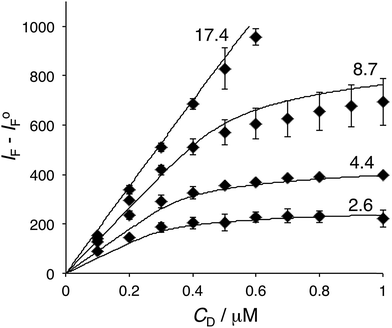 | ||
| Fig. 5 Experimental fluorescence intensity difference, IF − IoF, as a function of dye concentration, CD at several protein concentrations, CP (diamonds; the numbers associated with each curve identify the corresponding experimental values of CP in μM. The data are the average of 2–3 measurements ± SD). The solid curves represent IF − IoF calculated using eqn (6) and (7) with K = 0.12 μM−1, n = 10, SD = 0.40 μM, and proportionality constant, kF = 75 μM−1. Conditions: λex = 530 nm, λem = 538 nm; buffer: 10 mM TRIS (0.1 M NaCl, pH 7.4). | ||
In order to quantitatively describe the observed behavior, dye reversible aggregation could be represented as nD = Dn, with the following mass action law (eqn (4)):
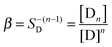 | (4) |
| IF − IoF = kF(R[PD] + [D] − [D]0) | (5) |
| CD = [D] + K[D]CP/(1 + K[D]) + nSD([D]/SD)n | (6) |
| CD = [D]0 + nSD([D]0/SD)n | (7) |
Conclusions
The interaction of several BODIPY dyes with BSA has been investigated using fluorescence spectroscopy. In the presence of BSA, krypto-BODIPY and click-BODIPY dyes exhibited a notable increase in their fluorescence intensities. In the case of the benzyl-triazole-containing BODIPY dye 3, a drastic increase in the fluorescence intensity was noted, yet the binding affinities for all three dyes towards BSA were found to be virtually the same. The fluorescence enhancement in this particular case demonstrates that BSA could play a dual role: (a) disaggregate the dye's aggregates and (b) subsequently bind monomeric BODIPY 3. Notably, a similar disaggregation phenomenon was also reported for aza-BODIPY dyes.20 Our results suggest that, at least in some cases, the fluorescence enhancement upon a dye–protein interaction might not be exclusively attributed to the binding event. Potentially the disaggregation of click-BODIPY dyes could be used as a detection event21 as well as a sensor for the hydrophobic surfaces of the proteins.14Acknowledgements
The project was partially supported by NIH R15AG038977 from the National Institute On Aging (to SVD) and ACS-PRF 47244-G4 (to OA).Notes and references
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- H. Sunahara, Y. Urano, J. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 5597–5604 CrossRef CAS PubMed.
- S.-l. Niu, C. Ulrich, P.-Y. Renard, A. Romieu and R. Ziessel, Chem. – Eur. J., 2012, 18, 7229–7242 CrossRef CAS PubMed.
- C. Bertucci and E. Domenici, Curr. Med. Chem., 2002, 9, 1463–1481 CrossRef CAS PubMed.
- J.-S. Lee, H. K. Kim, S. Feng, M. Vendrell and Y.-T. Chang, Chem. Commun., 2011, 47, 2339–2341 RSC.
- T. Komatsu, D. Oushiki, A. Takeda, M. Miyamura, T. Ueno, T. Terai, K. Hanaoka, Y. Urano, T. Mineno and T. Nagano, Chem. Commun., 2011, 47, 10055–10057 RSC.
- M. Vendrell, G. G. Krishna, K. K. Ghosh, D. Zhai, J.-S. Lee, Q. Zhu, Y. H. Yau, S. G. Shochat, H. Kim, J. Chung and Y.-T. Chang, Chem. Commun., 2011, 47, 8424–8426 RSC.
- J. C. Er, M. K. Tang, C. G. Chia, H. Liew, M. Vendrell and Y.-T. Chang, Chem. Sci., 2013, 4, 2168–2176 RSC.
- X. Duan, P. Li, P. Li, T. Xie, F. Yu and B. Tang, Dyes Pigm., 2011, 89, 217–222 CrossRef CAS.
- N. Dorh, S. Zhu, K. B. Dhungana, R. Pati, F.-T. Luo, H. Liu and A. Tiwari, Sci. Rep., 2015, 5, 18337, DOI:10.1038/srep18337.
- Yu. S. Marfin, E. L. Aleksakhina, D. A. Merkushev and E. V. Rumyantsev, J. Fluoresc., 2016, 26, 255–261 CrossRef CAS PubMed.
- N. W. Smith, A. Alonso, C. M. Brown and S. V. Dzyuba, Biochem. Biophys. Res. Commun., 2010, 391, 1455–1458 CrossRef CAS PubMed.
- J. Liang, B. Z. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798–2811 RSC.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2009 Search PubMed.
- R. Nagarajian, in Surfactant science and technology: retrospect and prospects, ed. L. Romsted, 2014, pp. 3–52 Search PubMed.
- X.-X. Zhang, Z. Wang, X. Yue, Y. Ma, D. O. Kiesewetter and X. Chen, Mol. Pharmaceutics, 2010, 10, 1910–1917 CrossRef PubMed.
- D. Zhai, W. Xu, L. Zhang and Y.-T. Chang, Chem. Soc. Rev., 2014, 43, 2402–2411 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of BODIPY dyes; details on the fluorescent experiments and sample preparations. See DOI: 10.1039/c6cp00420b |
| This journal is © the Owner Societies 2016 |