Superior adsorption of pharmaceutical molecules by highly porous BN nanosheets†
Dan
Liu
a,
Weiwei
Lei
*a,
Si
Qin
 a,
Karel D.
Klika
b and
Ying
Chen
*a
a,
Karel D.
Klika
b and
Ying
Chen
*a
aInstitute for Frontier Materials, Deakin University, Waurn Ponds, Victoria 3216, Australia. E-mail: weiwei.lei@deakin.edu.au; ian.chen@deakin.edu.au
bMolecular Structure Analysis, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, D-69009 Heidelberg, Germany
First published on 30th November 2015
Abstract
Highly porous boron nitride nanosheets (BNNSs) were tested as a re-usable adsorbent for the removal of pharmaceuticals from aqueous solution. The BNNSs exhibit both unprecedentedly high adsorption capacities and excellent recyclability while maintaining their high adsorption capacity by a simple regeneration process. These advantages render BNNSs a promising material for water remediation applications.
Due to the present high consumption of pharmaceuticals,1 drug compounds are often found in surface waters, groundwater, sediments, even drinking water,2–5 and environmental contamination by pharmaceuticals is of grave concern for aquatic ecosystems worldwide1 since pharmaceuticals are, by intent, biologically active compounds. For instance, psychotherapeutics in aquatic ecosystems can affect key behavior traits of organisms influencing their growth, reproduction, and survival.6 Entry into aquatic systems is mainly via waste water treatment plants receiving discharge from households, hospitals, livestock operations, and drug manufacturers.1,7 Various methods have been advocated for the removal of pharmaceuticals from contaminated water, including membrane separation,8 biological treatment,9 direct evaporation,10 electrodialysis,11 and catalytic oxidation.12 The use of adsorbents has grown due to their simplicity of use, high efficiency, and wide availability; bio-adsorbents, activated carbon, palygorskite, kaolite, clays, apatite, zeolite, and carbon nanotubes, have all been applied with positive results.13–21 Even waste plastic has been mooted7,22 as a potential mean of removing pharmaceuticals from waste water.
Following the discovery of graphene,23 two dimensional nanomaterials have attracted considerable attention. Since hexagonal boron nitride nanosheets (BNNSs) are isoelectronic with graphene, they are one of the most important group III–V materials with planar, trigonal sp2 bonding. In BNNSs, the B and N atoms substitute the C atoms in a graphite-like sheet with virtually indistinct atomic spacing. BNNSs possess a wide range of remarkable properties, including extremely high resistance to oxidation, low dielectric constant, high corrosion resistance, electrical-insulating properties, high mechanical strength, high surface area, and high thermal conductivity and stability.24–27 These properties avail BNNSs to a wide range of promising applications such as field nanoemitters, nanoelectronics, and composite reinforcement.28–30 Recently, innovative adsorptive applications have been explored for BNNSs, e.g., they have a strong performance in hydrogen adsorption and can effectively remove pollutants such as dyes, proteins, organic solvents, metal ions, and oils, from contaminated water.31–36 However, porous BNNSs have not been tested before as adsorbent for pharmaceuticals.
We have previously reported30 that porous BNNSs can be produced in bulk by a dynamic templating approach. Due to their nanosheet structure and high porosity with high specific surface area, these porous BNNSs exhibit superior adsorption performance for a range of oils, solvents, and dyes.32 Here we report the adsorption characteristics for four pharmaceuticals (Fig. 1), viz the antibiotics tetracycline (TC), chlortetracycline hydrochloride (CTC), ciprofloxacin (CIP), and norfloxacin (NOR). Both TC and CTC are frequently found at relatively high concentrations in the environment.37 The fluoroquinolones, another class of antibiotics, represented here by CIP and NOR, are also often found in surface waters. The adsorption kinetics, isotherms, and the rate of uptake were all evaluated to characterize the adsorption behaviors of these compounds towards porous BNNSs. To account for variability in conditions, adsorptions were also compared across a range of pHs, as was the practicality of the BNNSs by testing their recyclability.
Porous BNNSs were prepared32 by heating the complex formed between boron trioxide (B2O3) and guanidine hydrochloride at 1100 °C for 2 h under a cover of 15% H2 in N2 resulting in a high specific surface area of 1427 m2 g−1 and a pore size distribution ranging from 20 to 100 nm. Fig. 2 shows the TEM and high-magnification TEM images of these porous BNNSs whose morphology consist of well-defined nanosheets with a large number of pores in the range of 20–100 nm in diameter. Holes present in the BNNSs likely result from gas bubbles formed during production, but which also help facilitate the uptake of compounds.32
 | ||
| Fig. 2 (a) TEM image of a single porous BNNS. (b) High-magnification SEM image revealing the porous BNNS structure. | ||
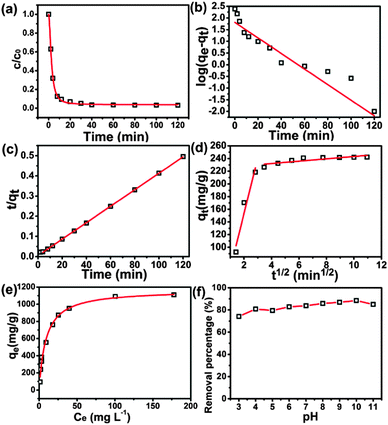
Adsorption kinetics for the drugs were facilitated by UV-vis monitoring to enable rate estimations for the possible reaction mechanism(s) involved in the adsorption process. Following the uptake of TC and CTC from 10 mL aliquots ([50 mg L−1]) by 2 mg of porous BNNSs, it was observed that adsorption was very rapid for the initial 12 min for TC and initial 8 min for CTC (Fig. 3a for CTC; see ESI,† Fig. ESI1a for TC). After 300 min and 120 min, ca. 99% adsorption was attained for TC and CTC, respectively. The experimental data were then fitted to pseudo-first-order, pseudo-second-order, and intraparticle diffusion models defined by the following equations:
| Qt = kidt1/2 intraparticle diffusion |
The linear fits for the model equations are presented in Fig. 3b–d for CTC (see ESI,† Fig. ESI1b–d for TC) with the resulting rate constants listed in Table 1. With very poor linear fits for the pseudo-first-order model but excellent fits for the pseudo-second-order model (R2 = 0.999 for both compounds), clearly the adsorption process follows the latter model for both CTC and TC and is suggestive of a chemisorption process.38 It is notable that the pseudo-second-order rate constants for CTC (4.08 × 10−3 g mg−1 min−1) and TC (4.09 × 10−3 g mg−1 min−1) are nearly identical, as one might expect for very similar compounds. The plot for the intraparticle diffusion model for CTC is presented in Fig. 3d (see ESI,† Fig. ESI1d for TC), whereby the two linear relationships observed indicate that two distinct stages are in effect in the adsorption process, viz boundary-layer diffusion and pore diffusion.39 The steeper gradient for the first adsorption step indicates that the rate of both CTC and TC removal are higher in the initial stages due to the ready availability of a large free surface area and multiple active adsorption sites. The lower gradient for the second step results from the extremely low concentration left in the solution leaving the adsorption to be dependent on the molecular diffusion of CTC and TC in the micropores of the BNNSs. Since this process is slow, it leads to a rate-limiting removal of the compounds.40
| Antibiotic | Pseudo-first-order | Pseudo-second-order | Intraparticle diffusion | |||||
|---|---|---|---|---|---|---|---|---|
| k 1 × 10−3 (min−1) | R 2 | k 2 × 10−3 (g mg−1 min−1) | R 2 | k id1 (g mg−1 min−1/2) | R 2 | k id2 (g mg−1 min−1/2) | R 2 | |
| TC | 7.51 | 0.819 | 4.09 | 0.999 | 56.93 | 0.955 | 2.61 | 0.966 |
| CTC | 33.3 | 0.908 | 4.08 | 0.999 | 86.96 | 0.933 | 1.84 | 0.915 |
| CIP | 14.5 | 0.712 | 12.4 | 0.999 | 28.99 | 0.944 | 0.34 | 0.908 |
| NOR | 24.1 | 0.887 | 10.2 | 0.997 | 34.63 | 0.966 | 0.95 | 0.896 |
To evaluate the adsorption capacity of the BNNSs, equilibrium adsorption isotherms were analyzed by the Langmuir adsorption model,35 defined by the equation:
| qe = qmbce/(1 + bce) |
The Langmuir isotherms (Fig. 3e for CTC; see ESI,† Fig. ESI1e for TC) indicate a maximum adsorption capacity, qm, corresponding to complete monolayer coverage, of 1170 mg g−1 (R2 = 0.991) for CTC and 284 mg g−1 (R2 = 0.986) for TC. These values agree well with measured values of 1110 mg g−1 for CTC (ce = [400 mg L−1]) and 272 mg g−1 for TC (ce = [120 mg L−1]).
The effect of pH over the range of 3–11 on the adsorption of TC from a solution ([120 mg L−1]) is shown in Fig. ESI1f (ESI†) whereby essentially, neglecting the likely anomalies at pH 3 and 8, no effect is discernible and the removal efficiency remains constant at ca. 60%. Thus pH has only a negligible influence on the interaction between TC and porous BNNSs. However, there is a slight, but clearly apparent, increase in adsorption over this same pH range for CTC ([250 mg L−1]), from 76% at pH 3 to 85% at pH 11 (Fig. 3f). This may simply be attributed to the deprotonation of the hydrochloride as CTC – but not TC – was used in such a state, and the likely reduction in hydrophilicity upon deprotonation of CTC to the free amine should render the CTC more amenable to adsorption by the BNSSs. Alternatively, Lewis acid–base interactions can occur between the porous BNNSs and CTC/TC induced by the known amino-affinitive behavior of BNNSs, which can also lead to enhancement of its adsorption properties,41 which would then also explain the increased adsorption of CTC hydrochloride with increasing pH as the amine is freed.
Following the uptake of CIP and NOR from 10 mL aliquots ([10 mg L−1]) by 2 mg of porous BNNSs, it was observed that adsorption was very rapid for both drugs for the initial 20 min (see ESI,† Fig. ESI2a and ESI3a for CIP and NOR, respectively). Both samples reached adsorption equilibrium after 120 min, with up to 80% adsorption for CIP and 95% adsorption for NOR. The experimental data were then similarly fitted to pseudo-first-order, pseudo-second-order, and intraparticle diffusion models (see ESI,† Fig. ESI2b–d and ESI3b–d for CIP and NOR, respectively, and Table 1). As previously, with very poor linear fits for the pseudo-first-order model but excellent fits for the pseudo-second-order model (R2 ≥ 0.997 for both compounds), the adsorption process also clearly follows the latter model for both CIP and NOR, and thus again, suggestive too of a chemisorption process38 being active. It is notable that the pseudo-second-order rate constants for CIP (12.4 × 10−3 g mg−1 min−1) and NOR (10.2 × 10−3 g mg−1 min−1) are likewise very close, as would be expected for very similar compounds. The plots for the intraparticle diffusion model (see ESI,† Fig. ESI2d and ESI3d for CIP and NOR, respectively) also reveal two linear relationships to again indicate that two distinct stages as well are in effect in the adsorption process for CIP and NOR, with similar conclusions as for TC and CTC regarding the underlying reasons for the observed behavior.
The Langmuir isotherms (see ESI,† Fig. ESI2e and ESI3e for CIP and NOR, respectively) indicate a qm of 206 mg g−1 (R2 = 0.981) for CIP and 174 mg g−1 (R2 = 0.995) for NOR.
The effect of pH over the range 3–11 on the adsorption was measured for CIP and NOR solutions ([120 mg L−1] for both). Both compounds exhibited similar trends with the removal efficiency dropping from ca. 50% at pH 3 to ca. 20% at the extreme pH of 11 (see ESI,† Fig. ESI2f and ESI3f for CIP and NOR, respectively). This diminution with increasing pH may be attributed to the progressive deprotonation of the acid group present in these molecules with the resulting carboxylate anion being rendered more hydrophilic, and thus less favoured towards adsorption by the BNSSs, in other words, overriding the π–π stacking interaction that is likely responsible for the adsorption of CIP and NOR by the porous BNNSs.42
The order of qm for the four antibiotics on porous BNNSs based on the Langmuir isotherms was CTC (1170 mg g−1) > TC (284 mg g−1) > CIP (206 mg g−1) > NOR (174 mg g−1). These capacities, significantly, are more than 10 times greater than those of the most adsorbent nanomaterials reported so far.43–45 The high adsorption capacities of the BNNSs for antibiotics is essentially due to their high specific surface area, large pore volume, and highly crumpled structure which exacerbates interfacial interactions.34 In addition, considering that the structures of the four molecules all encompass aromatic rings, we suggest that π–π interactions probably dominate the interaction between these molecules and the porous BNNSs due to the similarity of the structural features of BN networks and the aromatic rings of the organic molecules. Such interactions as a dominant driving force has always been used to explain the adsorption mechanism of aromatic molecules with graphene.42 Moreover, the adsorption capacities for CTC and TC are higher than for CIP and NOR due to the greater number of aromatic units in CTC and TC with four aromatic rings each compared to CIP and NOR with two aromatic rings each. Moreover, Lewis acid–base interactions can occur between the porous BNNSs and CTC and TC induced by the known amino-affinitive behavior of BNNSs, which can further enhance their adsorption properties.41
Finally, economical remediation demands that costly adsorbents be easily regenerated after the uptake of pollutants. To demonstrate the recyclability of the BNNSs, a comparison was made of the adsorption proficiency of regenerated porous BNNSs for CTC after multiple regeneration steps. The regeneration process consisted simply of combustion treatment in air and it was serendipitously found that the regenerated BNNSs were still able to remove ca. 82% of the CTC even after ten successive regenerations (see ESI,† Fig. ESI4). Of note however, the surface area of regenerated BNNSs was found to decrease to 940 m2 g−1 (see ESI,† Fig. ESI5) while the pore distribution didn't change. It has been suggested that carbonaceous particulate matter present after a regeneration process can attach to the surface of regenerated BNNSs,32 which would account for the decreased BET surface area and slight reduction in adsorption capacity. The excellent recyclability of these porous BNNSs thus renders them potentially cost effective when using them as adsorbents for practical remediation purposes.
In summary, porous BNNSs have been evaluated with respect to the adsorption kinetics of TC, CTC, CIP, and NOR, and for which they showed high adsorption capacities based on Langmuir isotherms of 1170 mg g−1 (CTC), 284 mg g−1 (TC), 206 mg g−1 (CIP), and 174 mg g−1 (NOR). All four antibiotics from two different classes exhibited adsorption processes on porous BNNSs which strongly obeyed pseudo-second-order kinetics, i.e., the adsorption rate is controlled by chemical interaction.35 In addition, plots of qtvs. t1/2 for the intraparticle diffusion model revealed two linear sections in each case for all four compounds, thus indicating that two distinct stages are in effect in the adsorption process, viz boundary-layer diffusion and pore diffusion.39
While the adsorption of TC and CTC on porous BNNSs exhibited negligible or near negligible pH dependencies, the adsorption of both CIP and NOR diminished progressively with increasing pH. Nevertheless, good adsorption performance over a wide pH range was observed in all cases. In addition, the porous BNNSs exhibited excellent recyclability after antibiotic adsorption and regeneration. All these features endow porous BNNSs with great potential for practical application as an adsorbent for water purification and treatment in addition to the demonstrated applications of kaolite, palygorskite, clays, bio-adsorbents, apatite, zeolite, activated carbon, and carbon nanotubes for the removal of pharmaceuticals from contaminated waste water. It can be well anticipated that a combined or holistic approach,7,22 perhaps a high-low tech one, might be required to cover all cases in real world applications.
Notes and references
- R. Triebskorn, K. Berg, I. Ebert, M. Frey, D. Jungmann, J. Oehlmann, M. Oetken, F. Sacher, M. Scheurer, H. Schmieg, S. Schwarz and H.-R. Köhler, Environ. Sci. Technol., 2015, 49, 2594 CrossRef CAS PubMed.
- L. Ji, W. Chen, L. Duan and D. Zhu, Environ. Sci. Technol., 2009, 43, 2322 CrossRef CAS PubMed.
- H. Y. Sun, X. Shi, J. D. Mao and D. Q. Zhu, Environ. Toxicol. Chem., 2010, 29, 1934 CAS.
- V. Diwan, A. J. Tamhankar, M. Aggarwal, S. Sen, R. K. Khandal and C. Stålsby Lundborg, Science, 2009, 97, 1752 CAS.
- K. Fent, A. A. Weston and D. Caminada, Aquat. Toxicol., 2006, 76, 122 CrossRef CAS PubMed.
- T. Brodin, J. Fick, M. Jonsson and J. Klaminder, Science, 2013, 339, 814 CrossRef CAS PubMed.
- K. D. Klika, Adv. Water Res. Prot., 2014, 2, 21 Search PubMed.
- K. Kimura, G. Amy, J. E. Drewes, T. Heberer, T. U. Kim and Y. Watanabe, J. Membr. Sci., 2003, 227, 113 CrossRef CAS.
- K. E. Conn, L. B. Barber, G. K. Brown and R. L. Siegrist, Environ. Sci. Technol., 2006, 40, 7358 CrossRef CAS PubMed.
- W. Pronk and D. Kone, Desalination, 2009, 248, 360 CrossRef CAS.
- M. C. Dodd, S. Zuleeg, U. von Gunten and W. Pronk, Environ. Sci. Technol., 2008, 42, 9329 CrossRef CAS PubMed.
- A. Ghauch, A. Tuqan and H. A. Assi, Environ. Pollut., 2009, 157, 1626 CrossRef CAS PubMed.
- P. H. Chang, Z. H. Li, T. L. Yu, S. Munkhbayer, T. H. Kuo, Y. C. Hung, J. S. Jean and K. H. Lin, J. Hazard. Mater., 2009, 165, 148 CrossRef CAS PubMed.
- C. Gu and K. G. Karthikeyan, Environ. Sci. Technol., 2005, 39, 2660 CrossRef CAS PubMed.
- Z. H. Li, H. Hong, L. B. Liao, C. J. Ackley, L. A. Schulz, R. A. Macdonald, A. L. Mihelich and S. M. Emard, Colloids Surf., B, 2011, 88, 339 CrossRef CAS PubMed.
- Q. F. Wu, Z. H. Li, H. L. Hong, K. Yin and L. Y. Tie, Appl. Clay Sci., 2010, 50, 204 CrossRef CAS.
- C. J. Wang, Z. H. Li and W. T. Jiang, Appl. Clay Sci., 2011, 53, 723 CrossRef CAS.
- H. M. Ötker and I. Akmehmet-Balcıoğlu, J. Hazard. Mater., 2005, 122, 251 CrossRef PubMed.
- H. C. Zhang and C.-H. Huang, Chemosphere, 2007, 66, 1502 CrossRef CAS PubMed.
- S. A. C. Carabineiro, T. Thavorn-Amornsri, M. F. R. Pereira and J. L. Figueiredo, Water Res., 2011, 45, 4583 CrossRef CAS PubMed.
- L. L. Ji, W. Chen, S. R. Zheng, Z. Y. Xu and D. Q. Zhu, Langmuir, 2009, 25, 11608 CrossRef CAS PubMed.
- K. D. Klika, Environ. Sci. Technol., 2013, 47, 10111 CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- L. Song, L. Ci, H. Lu, P. B. Sorokin, C. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209 CrossRef CAS PubMed.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889 CrossRef CAS.
- X. Wang, C. Zhi, L. Li, H. Zeng, C. Li, M. Mitome, D. Golberg and Y. Bando, Adv. Mater., 2011, 23, 4072 CrossRef CAS PubMed.
- Q. Weng, X. Wang, C. Zhi, Y. Bando and D. Golberg, ACS Nano, 2013, 7, 1558 CrossRef CAS PubMed.
- W. Lei, D. Portehault, R. Dimova and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 7121 CrossRef CAS PubMed.
- R. Gao, L. Yin, C. Wang, Y. Qi, N. Lun, L. Zhang, Y.-X. Liu, L. Kang and X. Wang, J. Phys. Chem. C, 2009, 113, 15160 CAS.
- Y. C. Zhu, Y. Bando, L. W. Yin and D. Golberg, Nano Lett., 2006, 6, 2982 CrossRef CAS PubMed.
- J. Li, X. Xiao, X. Xu, J. Lin, Y. Huang, Y. Xue, P. Jin, J. Zou and C. Tang, Sci. Rep., 2013, 3, 3208 Search PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- D. Liu, W. Lei, S. Qin and Y. Chen, Sci. Rep., 2014, 4, 4453 Search PubMed.
- W. Lei, D. Lui and Y. Chen, Adv. Mater. Interfaces, 2015, 2, 1400529 Search PubMed.
- J. Li, J. Lin, X. Xu, X. Zhang, Y. Xue, J. Mi, Z. Mo, Y. Fan, L. Hu, X. Yang, J. Zhang, F. Meng, S. Yuan and C. Tang, Nanotechnology, 2013, 24, 155603 CrossRef PubMed.
- W. W. Lei, H. Zhang, Y. Wu, B. Zhang, D. Liu, S. Qin, Z. W. Liu, L. M. Liu, Y. M. Ma and Y. Chen, Nano Energy, 2014, 6, 219 CrossRef CAS.
- S. K. Khetan and T. J. Collins, Chem. Rev., 2007, 107, 2319 CrossRef CAS PubMed.
- Y.-S. Ho, J. Hazard. Mater., 2006, 136, 681 CrossRef CAS PubMed.
- X. Luo, C. Wang, L. Wang, F. Deng, S. Luo, X. Tu and C. Au, Chem. Eng. J., 2013, 220, 98 CrossRef CAS.
- Y. H. Li, Q. J. Du, T. H. Liu, J. K. Sun, Y. Q. Jiao, Y. Z. Xia, L. H. Xia, Z. H. Wang, W. Zhang, K. L. Wang, H. W. Zhu and D. H. Wu, Mater. Res. Bull., 2012, 47, 1898 CrossRef CAS.
- L. Ji, W. Chen, Z. Xu, S. Zheng and D. Zhu, J. Environ. Qual., 2013, 42, 191 CrossRef CAS PubMed.
- Y. Chao, W. Zhu, X. Wu, F. Hou, S. Xun, P. Wu, H. Ji, H. Xu and H. Li, Chem. Eng. J., 2014, 243, 60 CrossRef CAS.
- Y. Lin, S. Xu and L. Jia, Chem. Eng. J., 2013, 225, 679 CrossRef CAS.
- Y. Tang, H. Guo, L. Xiao, S. Yu, N. Gao and Y. Wang, Colloids Surf., A, 2013, 424, 74 CrossRef CAS.
- X. Zhu, Y. Liu, F. Qian, C. Zhou, S. Zhang and J. Chen, Bioresour. Technol., 2014, 154, 209 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Description of materials and methods; graphs for the adsorption kinetics, isotherms, rates of uptake, and uptake variations with pH for all compounds (TC, CIP, and NOR); and a bar graph depicting the recyclability of the BNNSs. See DOI: 10.1039/c5cp06399j |
| This journal is © the Owner Societies 2016 |