h-BN nanosheets as simple and effective additives to largely enhance the activity of Au/TiO2 plasmonic photocatalysts†
Y.
Ide
*ab,
K.
Nagao
b,
K.
Saito
b,
K.
Komaguchi
c,
R.
Fuji
d,
A.
Kogure
e,
Y.
Sugahara
*bfg,
Y.
Bando
a and
D.
Golberg
a
aInternational Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan. E-mail: IDE.Yusuke@nims.go.jp
bGraduate School of Creative Science and Engineering, Waseda University, 1-6-1 Nishiwaseda, Shinjuku-ku, Tokyo 169-8050, Japan
cGraduate School of Engineering, Department of Applied Chemistry, Hiroshima University, Kagamiyama 1-4-1, Higashi-Hiroshima 739-8527, Japan
dShimadzu Cooperation, 380-1 Horiyamashita, Hadano-shi, Kanagawa 259-1304, Japan
eShimadzu Techno-Research, INC, 380-1 Horiyamashita, Hadano-shi, Kanagawa 259-1304, Japan
fDepartment of Applied Chemistry, School of Advanced Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555, Japan
gKagami Memorial Research Institute for Materials Science and Technology, Waseda University, 2-8-26 Nishiwaseda, Shinjuku-ku, Tokyo 169-0051, Japan
First published on 4th November 2015
Abstract
The activity of Au nanoparticle-loaded P25 TiO2 (Au/P25) plasmonic photocatalysts, evaluated by the oxidative decomposition of formic acid in water under visible light irradiation, was enhanced up to 3 times by simply mixing Au/P25 with photocatalytically inactive h-BN nanosheets as a result of electron transfer from photoexcited Au/TiO2 to the h-BN nanosheets and retardation of the charge recombination.
Visible light-driven photocatalysts are attracting increasing attention for the potential applications of their efficient utilization of solar light or indoor illumination for environmental purification, fuel production and fine chemical syntheses.1–3 TiO2 is one of the most promising photocatalysts due to its combined advantages of low cost with long-term chemical stability and negligible toxicity. As it absorbs only UV light occupying 3–5% of the incident solar spectrum, the visible-light response of TiO2 is being actively investigated using various approaches, including heteroelemental doping4 and hybridization (or grafting) with metals (metal ions)5 and metal oxides6 and the introduction of disordered phases.7 Au nanoparticle-loaded TiO2 (Au/TiO2) has been the most widely studied visible light-responsive photocatalyst,8 since Tatsuma et al. demonstrated that visible light excitation of the localized surface plasmon resonance of Au/TiO2 caused electron injection from Au to TiO2.8a So far, the performances of Au/TiO2 plasmonic photocatalysts have been optimized mainly by the catalysts' architectures (such as TiO2 crystalline forms9 and Au particle sizes10 and locations11) and substantially enhanced by the deposition of co-catalysts such as Pt.12
h-BN, a structural analogue of graphite, possesses useful properties such as high chemical and thermal stability and excellent thermal conductivity, and it thus offers a wide range of potential industrial applications.13 h-BN is an insulator with a wide band gap (up to 7.1 eV depending on the experimental method13a,14). Some recent studies have demonstrated theoretically and experimentally, however, that depending on its morphology, nanostructured h-BN shows semiconducting properties15 or electronic transport comparable to metals or metal oxides.16,17 We have recently reported that Au/TiO2 supported on h-BN nanosheets shows enhanced plasmonic activity as compared with Au/TiO2.17c An electron transfer from Au/TiO2 to h-BN to retard charge recombination on Au/TiO2 was proposed as one reason for the enhancement.
It has been reported that simple mixing (physical mixing in reaction media) of different kinds of TiO2 particles (including TiO2-based photocatalysts) sometimes causes synergetically enhanced photocatalytic efficiency as a result of electron transfer from the photoexcited particles to other particles to retard charge recombination.18 Accordingly, mixing of state-of-the-art photocatalysts with abundant or conventional materials can be a simple, more cost-effective and more versatile way to achieve highly efficient photocatalytic reactions as compared with the design of time-consuming and costly new photocatalysts.19 Herein we report that Au/TiO2 shows considerably enhanced plasmonic activity when simply mixed with h-BN nanosheets. We show that mixing Au/TiO2 with h-BN nanosheets can enhance plasmonic activity much more efficiently than hybridization.17c
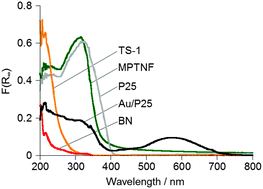
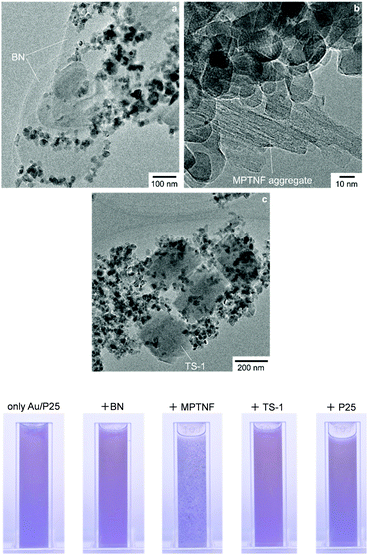
In this study, we used P25 as TiO2 to prepare Au/P25, one of the most active Au/TiO2 plasmonic photocatalysts with respect to oxidation reactions.11c As shown in Fig. 1a, Au nanoparticles 5–10 nm in size were located at the interfaces of TiO2 (anatase and rutile) particles, rather than on the surface of TiO2 particles. This Au location in Au/P25 has been suggested to be the key to their high charge separation efficiency and thus to high photocatalytic performance.11c A hybrid of Au/TiO2 with h-BN nanosheets (Au/TiO2/BN)17c was also used as a photocatalyst for comparison. h-BN nanosheets (BN hereafter) were prepared according to our previous report17a and used as an additive (Fig. 1b and c). In addition to BN, a microporous titanate nanofiber (MPTNF), a newly developed and photocatalytically inactive TiO2-based compound (Fig. 1d)20 that can enhance the activity of P25 when simply mixed with P25,19a a titanosilicate zeolite (TS-1, Fig. 1e) which is known as a single-site photocatalyst,2c,21 and P25 were used as additives to better understand the effects of electronic band structures and particle morphologies of additives on the photocatalytic activities of mixtures. Since none of the additives absorbed more than a negligible amount of visible light, as confirmed by the UV-vis spectra (Fig. 2), they were thought not to reduce visible light absorption by Au/P25.
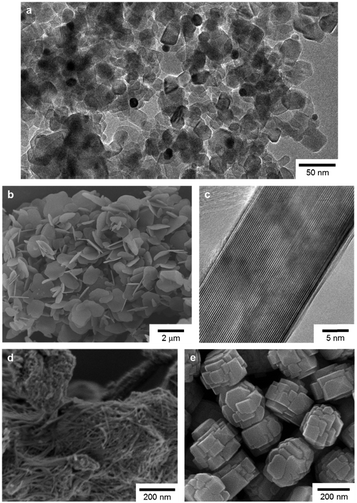 | ||
| Fig. 1 (a) TEM image of Au/P25, (b) SEM and (c) cross-sectional high resolution TEM images of BN, and SEM images of (d) MPTNF and (e) TS-1. | ||
Photocatalytic performance was evaluated by oxidative decomposition of formic acid in water to evolve CO2, a representative reaction to test the performance of photocatalysts for the removal of organic pollutants.19a,22,23 Particles of Au/P25 and BN (or other additives) were mixed into the formic acid solution (O2 saturated) by ultrasonication and subsequent stirring under light-shielded conditions, and the dispersion or mixture was photoirradiated (λ > 420 nm) under stirring. The amount of evolved CO2 required for formic acid decomposition on all the samples increased linearly with the irradiation time. It should be noted that formic acid adsorption on both the photocatalysts and the additives was negligible under the present testing conditions (leading us to start photoirradiation immediately after the mixing).19a In the case of photocatalytic decomposition of an organic dye in water by Au/TiO2/BN, by contrast, the enhanced adsorption owing to BN support was another reason for the enhanced plasmonic activity.17c
Fig. 3 shows CO2 evolution rates for P25, Au/P25 and Au/P25 additive mixtures at different mixing ratios. Because P25 did not evolve CO2 at all under the present condition, the CO2 evolution observed for Au/P25 resulted from the localized surface plasmon resonance; visible light excitation of Au/P25 caused electron injection from Au into the conduction band of P25 to create electron-deficient Au that could oxidize formic acid (electrons on P25 were reduced by O2 to be consumed).10 Au/P25-BN mixtures showed higher activity than Au/P25. Since BN was completely inactive for the reaction, synergy effects of the presence of both Au/P25 and BN on the photocatalytic reaction were revealed. The enhancement of the activity of Au/P25 was up to 3 times depending on the amount of BN added and comparable to that observed when noble metal co-catalysts were deposited on Au/TiO2.12 Au/P25-BN mixtures were reusable for further reactions, moreover, with no reduction in their original activities.
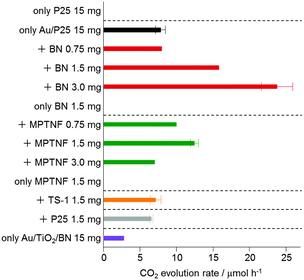 | ||
| Fig. 3 Photocatalytic activities for the oxidation of formic acid in water to CO2 over different samples under visible light irradiation (λ > 420 nm). | ||
Although MPTNF also synergetically enhanced the activity of Au/P25 plasmonic photocatalysts, the enhancement was considerably more limited than that observed upon addition of BN. TS-1 and P25, on the other hand, had no effect on the activity of Au/P25 (Fig. 3).
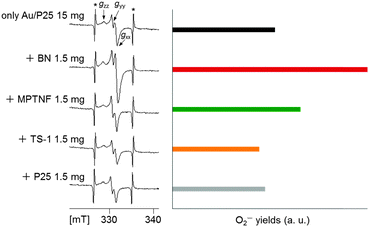
We investigated the possible reasons for the considerably higher activity of the Au/P25-BN mixture than that of other mixtures. The photocatalytic oxidative decomposition of organic compounds into CO2 proceeds efficiently with the consumption (reduction) of excited electrons by molecular O2 to allow superoxide-type oxygen anions (O2−) and then retard electron–hole recombination.24 Here, we investigated possible electron transfer from photoexcited Au/P25 to the additives by monitoring O2− with electron spin resonance (ESR) analysis (Fig. 4). When irradiated with visible light (λ > 420 nm) in the presence of molecular O2, Au/P25 created signals due to O2− formed by the reduction of O2 with the photoexcited electrons.11c,25 When an Au/P25-BN mixture was irradiated under the identical conditions, the O2− yield increased remarkably. The O2− yield for the Au/P25-MPTNF mixture increased only slightly, on the other hand, and those for the Au/P25-TS-1 and Au/P25-P25 mixtures were closely similar to that of Au/P25 without additives. These results indicate a unique and highly efficient electron transfer from photoexcited Au/P25 to BN. This electron transfer is thermodynamically favourable if the conduction band potential of BN is more positive than that of P25 (anatase component11c). Though the conduction band potential of BN is unknown, calculations26 show the conduction band potential of semihydrogenated h-BN monolayered nanosheets to be similar to that of TiO2 (−0.25 V vs. NHE for anatase27). Since the Au/P25-BN mixture produced a larger amount of O2− than Au/P25, BN also produced O2− and the conduction band potential of BN should thus have a negativity greater than −0.13 V (vs. NHE) in terms of O2/O2− reduction potential.27 Therefore, the conduction band potential of BN seems to lie between −0.13 V (vs. NHE) and −0.25 V (vs. NHE). Electron transfer from photoexcited Au/P25 to MPTNF and P25 is also thermodynamically favourable (that to TS-1 is unfavourable) on the basis of the conduction band potentials of Au/P25 and the additives.14a,28 Accordingly, one possible reason for the higher activity of the Au/P25-BN mixture than that of the other mixtures is the more efficient electron transfer between Au/P25 and BN due to a larger conduction band potential difference between them. (We cannot rule out the possibility of direct interactions between the Au of Au/P25 and BN to enhance the plasmonic activity of the Au nanoparticles itself8b).
Another possible reason for the greatly enhanced activity of the Au/P25-BN mixture are the homogeneous mixing conditions of individual particles. Fig. 5 (top) shows the TEM images of the Au/P25 mixtures with BN, MPTNF and TS-1 after evaporation of water. (The TEM image of the Au/P25-P25 mixture is not shown because it was difficult to differentiate the individual component particles in the mixture). For the Au/P25-BN mixture, numerous Au/P25 particles were homogeneously located on a BN particle; each particle was in good contact with the others, which correlates with efficient electron transfer between the particles. The BN particles were large and possessed platelike morphologies (Fig. 1b and c), and they seemed to be compatible with relatively small and platelike Au/P25 particles. (Au/P25 particles were also located on the edges of the BN plates, presumably due to the fact that h-BN nanosheets sometimes have (002) plane edges terminated with OH groups that can interact with OH groups on the TiO2 surface17d). The TS-1 particles were less homogeneously mixed with the Au/P25 particles than the BN particles, probably because of their cubic shape and uneven surfaces (Fig. 1e). MPTNF particles (in which the diameter of each nanofiber is ca. 10 nm16), on the other hand, were highly aggregated, and MPTNF and Au/P25 particles could thus not be homogeneously mixed. As shown in Fig. 5 (bottom), moreover, the Au/P25-MPTNF mixture formed sub-mm-scale aggregated particles in water (probably due to a strong interaction between MPTNF agglomerates and Au/P25). It is difficult for light to penetrate effectively into such large aggregates; the Au/P25-MPTNF mixtures consequently seemed to show lower activity when larger amounts of MPTNF were added, unlike the Au/P25-BN mixtures (Fig. 3).
It is worth noting that Au/TiO2/BN showed considerably lower plasmonic activity than the Au/P25-BN mixtures and lower activity than Au/P25 alone with the same amount of photocatalysts (Fig. 1). This is due mainly to the facts that Au/P25 shows much higher plasmonic activity than the Au/TiO2 (anatase) component of Au/TiO2/BN as a result of higher charge separation efficiency11c and that only a small amount (ca. 5 wt%) of Au/TiO2 is contained in Au/TiO2/BN.17c It is difficult to deposit P25 particles and a larger amount of anatase fine particles on BN for synthesizing Au/TiO2/BN hybrids with enhanced plasmonic activity. It is also difficult to use a larger amount of Au/TiO2/BN in the present photocatalytic reaction because of its bulkiness (lightness) and resistance to wetting with water. The availability of this highly active Au/P25 in adequate amounts is thus a great advantage of the present simple method of mixing with BN over the previous hybridization approach.
In summary, we have reported that Au/TiO2 plasmonic photocatalysts (Au/P25) show significantly enhanced activity when simply mixed with h-BN nanosheets. This enhancement stemmed from an efficient electron transfer from photoexcited Au/TiO2 to the h-BN nanosheet additive to retard the charge recombination of the plasmonic photocatalyst. A wide variety of nanostructured h-BN materials with different properties, including visible light absorption,17d are available. The mixing of TiO2-based photocatalysts with h-BN materials is a challenge worth investigating not only to enhance efficiency but also to respond to visible light in a simple and economically beneficial manner.
Notes and references
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- (a) A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC; (b) A. Dhaksshinamoorthy, S. Navalon, A. Corma and H. Garcia, Energy Environ. Sci., 2012, 5, 9217 RSC; (c) M. Kohsuke, H. Yamashita and M. Anpo, RSC Adv., 2012, 2, 3165 RSC.
- (a) Y. Shiraishi and T. Hirai, J. Photochem. Photobiol., C, 2008, 9, 157 CrossRef CAS; (b) X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2014, 43, 473 RSC.
- (a) R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed; (b) M. Anpo and M. Takeuchi, J. Catal., 2003, 216, 505 CrossRef CAS.
- (a) H. Tada, T. Kiyonaga and S. Naya, Chem. Soc. Rev., 2009, 38, 1849 RSC; (b) H. Irie, S. Miura, R. Nakamura and K. Hashimoto, Chem. Lett., 2008, 37, 252 CrossRef CAS.
- (a) F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294 RSC; (b) J. L. Gunjakar, I. Y. Kim, J. M. Lee, Y. K. Jo and S.-J. Hwang, J. Phys. Chem. C, 2014, 118, 3847 CrossRef CAS.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746 CrossRef CAS PubMed.
- (a) Y. Tian and T. Tatsuma, J. Am. Chem. Soc., 2005, 127, 7632 CrossRef CAS PubMed; (b) as a very recent review: H. Cheng, K. Fuku, Y. Kuwahara, K. Mori and H. Yamashita, J. Mater. Chem. A, 2015, 3, 5244 RSC.
- (a) K. Kimura, S. Naya, Y. Jin-Nouchi and H. Tada, J. Phys. Chem. C, 2012, 116, 7111 CrossRef CAS; (b) A. Tanaka, A. Ogino, M. Iwaki, K. Hashimoto, A. Ohnuma, F. Amano, B. Ohtani and H. Kominami, Langmuir, 2012, 28, 13105 CrossRef CAS PubMed; (c) Z. Bian, T. Tachikawa, P. Zhang, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2014, 136, 458 CrossRef CAS PubMed.
- (a) E. Kowalska, R. Abe and B. Ohtani, Chem. Commun., 2009, 241 RSC; (b) E. Kowalska, O. O. P. Mahaney, R. Abe and B. Ohtani, Phys. Chem. Chem. Phys., 2010, 12, 2344 RSC.
- (a) S. Naya, A. Inoue and H. Tada, J. Am. Chem. Soc., 2010, 132, 6292 CrossRef CAS PubMed; (b) Y. Ide, M. Matsuoka and M. Ogawa, J. Am. Chem. Soc., 2010, 132, 16762 CrossRef CAS PubMed; (c) D. Tsukamoto, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, J. Am. Chem. Soc., 2012, 134, 6309 CrossRef CAS PubMed.
- (a) A. Tanaka, S. Sakaguchi, K. Hashimoto and H. Kominami, ACS Catal., 2013, 3, 79 CrossRef CAS; (b) T. Kume, S. Naya and H. Tada, J. Phys. Chem. C, 2014, 118, 26887 CrossRef.
- (a) D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979 CrossRef CAS PubMed; (b) Y. Lin and J. W. Connell, Nanoscale, 2012, 4, 6908 RSC.
- V. L. Solozhenko, A. G. Lazarenko, J. P. Petitet and A. V. Kanaev, J. Phys. Chem. Solids, 2001, 62, 1331 CrossRef CAS and references therein.
- H. B. Zeng, C. Y. Zhi, Z. H. Zhang, X. L. Wei, X. B. Wang, W. L. Guo, Y. Bando and D. Golberg, Nano Lett., 2010, 10, 5049 CrossRef CAS PubMed.
- (a) A. Lyalin, A. Nakayama, K. Uosaki and T. Taketsugu, J. Phys. Chem. C, 2013, 117, 21359 CrossRef CAS; (b) K. Uosaki, G. Elumalai, H. Noguchi, T. Masuda, A. Lyalin, A. Nakayama and T. Taketsugu, J. Am. Chem. Soc., 2014, 136, 6542 CrossRef CAS PubMed.
- (a) C. Tang, J. Li, Y. Bando, C. Zhi and D. Golberg, Chem. – Asian J., 2010, 5, 1220 CrossRef CAS PubMed; (b) X. Fu, Y. Hu, Y. Yang, W. Liu and S. Chen, J. Hazard. Mater., 2013, 244–245, 102 CrossRef CAS PubMed; (c) Y. Ide, F. Liu, J. Zhang, N. Kawamoto, K. Komaguchi, Y. Bando and D. Golberg, J. Mater. Chem. A, 2014, 2, 4150 RSC; (d) Q. Weng, Y. Ide, X. Wang, C. Zhang, X. Jiang, Y. Xue, P. Dai, K. Komaguchi, Y. Bando and D. Golberg, Nano Energy, 2015, 16, 19 CrossRef CAS.
- (a) T. Ohno, K. Tokieda, S. Higashida and M. Matsumura, Appl. Catal., A, 2003, 244, 383 CrossRef CAS; (b) C. Wang, C. Böttcher, D. W. Bahnemann and J. K. Dohrmann, J. Mater. Chem., 2003, 13, 2322 RSC; (c) Y. Park, W. Kim, D. Monllor-Satoca, T. Tachikawa, T. Majima and W. Choi, J. Phys. Chem. Lett., 2013, 4, 189 CrossRef CAS PubMed.
- (a) Y. Ide and K. Komaguchi, J. Mater. Chem. A, 2015, 3, 2541 RSC; (b) T. Nakato, T. Fujita and E. Mouri, Phys. Chem. Chem. Phys., 2015, 17, 5547 RSC.
- H. Hattori, Y. Ide and T. Sano, J. Mater. Chem. A, 2014, 2, 16381 CAS.
- N. Tsunoji, Y. Ide, Y. Yagenji, M. Sadakane and T. Sano, ACS Appl. Mater. Interfaces, 2014, 6, 4616 CAS.
- O.-O. Prieto-Mahaney, N. Murakami, R. Abe and B. Ohtani, Chem. Lett., 2009, 38, 238 CrossRef CAS.
- H. Kominami, A. Tanaka and K. Hashimoto, Chem. Commun., 2010, 46, 1287–1289 RSC.
- Y. Nosaka, Y. Yamashita and H. Fukuyama, J. Phys. Chem. B, 1997, 101, 5822 CrossRef CAS.
- M. Chiesa, M. C. Paganini, S. Livraghi and E. Giamello, Phys. Chem. Chem. Phys., 2013, 15, 9435 RSC.
- X. Li, J. Zhao and J. Yang, Sci. Rep., 2013, 3, 1858 Search PubMed.
- H. P. Maruska and A. K. Ghosh, Sol. Energy, 1978, 20, 443 CrossRef CAS.
- T. Arai, M. Yanagida, Y. Konishi, Y. Iwasaki, H. Sugihara and K. Sayama, J. Phys. Chem. C, 2007, 111, 7574 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c5cp05958e |
| This journal is © the Owner Societies 2016 |