Electronic energy and electron transfer processes in photoexcited donor–acceptor dyad and triad molecular systems based on triphenylene and perylene diimide units†
Abstract
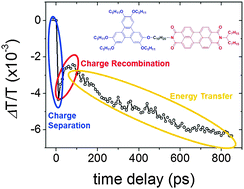
We investigate the photophysical properties of organic donor–acceptor dyad and triad molecular systems based on triphenylene and perylene diimide units linked by a non-conjugated flexible bridge in solution using complementary optical spectroscopy techniques. When these molecules are diluted in dichloromethane solution, energy transfer from the triphenylene to the perylene diimide excited moieties is evidenced by time-resolved fluorescence measurements resulting in a quenching of the emission from the triphenylene moieties. Simultaneously, another quenching process that affects the emission from both donor and acceptor units is observed. Solution ultrafast transient absorption measurements provide evidence of photo-induced charge transfer from either the donor or the acceptor depending upon the excitation. Overall, the analysis of the detailed time-resolved spectroscopic measurements carried out in the dyad and triad systems as well as in the triphenylene and perylene diimide units alone provides useful information both to better understand the relations between energy and charge transfer processes with molecular structures, and for the design of future functional dyad and triad architectures based on donor and acceptor moieties for organic optoelectronic applications.


 Please wait while we load your content...
Please wait while we load your content...