Fluid transition layer between rigid solute and liquid solvent: is there depletion or enrichment?
Abstract
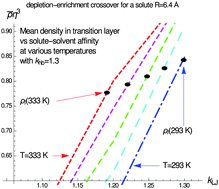
The fluid layer between solute and liquid solvent is studied by combining the density functional theory with the probabilistic hydrogen bond model. This combination allows one to obtain the equilibrium distribution of fluid molecules, taking into account the hydrogen bond contribution to the external potential whereto they are subjected near the solute. One can find the effective width of the fluid solvent–solute transition layer and fluid average density in that layer, and determine their dependence on temperature, solvent–solute affinity, vicinal hydrogen bond (hb) energy alteration ratio, and solute radius. Numerical calculations are performed for the solvation of a plate and spherical solutes of four different radii in two model solvents (associated liquid and non-associated one) in the temperature range from 293 K to 333 K for various solvent–solute affinities and hydrogen bond energy alteration ratios. The predictions of our model for the effective width and average density of the transition layer are consistent with experiments and simulations. The small-to-large crossover lengthscale for hydrophobic hydration is expected to be about 3–5 nm. Remarkably, characterizing the transition layer with the average density, one can observe that for small hydrophobes, the transition layer becomes enriched with rather than depleted of fluid when the solvent–solute affinity and hb-energy alteration ratio become large enough. The boundary values of solvent–solute affinity and hb-energy alteration ratio, needed for the “depletion-to-enrichment” crossover (in the smoothed density sense), are predicted to decrease with increasing temperature.


 Please wait while we load your content...
Please wait while we load your content...