Open Access Article
Open Access ArticleStructural transformations in crystals induced by radiation and pressure. Part 4. The complex influence of high pressure on the path and kinetics of the [2 + 2] photodimerization†
J.
Bąkowicz
* and
I.
Turowska-Tyrk
Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeże Wyspiańskiego 27, 50-370 Wrocław, Poland. E-mail: julia.bakowicz@pwr.edu.pl; Fax: +48 71 320 33 64
First published on 25th October 2016
Abstract
The results from monitoring structural changes brought about by the [2 + 2] photodimerization of 2-benzyl-5-benzylidenecyclopentanone (BBCP) in crystals at high pressure were presented. This is one of the two first examples of such studies for an intermolecular reaction. The influence of pressure of 0.5 GPa on the extent of changes caused by the photochemical reaction in the cell volume, in the molecular orientation and in the distance between reactive atoms is the same or similar to for 0.1 MPa of pressure, however, the character of the changes in the above-mentioned distance is different. The reason for the observed close similarity between the reaction path at 0.1 MPa and 0.5 GPa is a very good fit for dimer molecules to the crystal lattice of the monomer. The influence of high pressure itself on (a) the volume of the unit cell, (b) the volume of the free space in the unit cell, (c) the geometry of intermolecular interactions and (d) the distance between directly reacting atoms of two adjacent molecules was also described. The reaction rate is greater at 0.7 GPa than at 0.5 GPa; however, the difference is small. This was explained by the consistency between structural changes that came about by the photochemical reaction and by high pressure.
Introduction
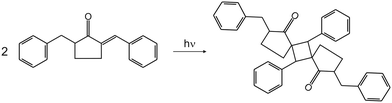
From the scientific literature, it is known that 2-benzyl-5-benzylidenecyclopentanone (BBCP) under UV-vis radiation under ambient conditions undergoes the [2 + 2] photodimerization in crystals and that this reaction can proceed in a homogeneous manner.1–5 The formula of BBCP and the equation of the [2 + 2] photodimerization are presented in Scheme 1. The path of the structural changes created by this photochemical reaction in crystals under ambient conditions was studied in the past.3,4 It was discovered that molecules of the reactant (monomer) and of the product (dimer) change their position during the phototransformation of crystals. The product molecules move towards the position occupied in the pure product crystals and the reactant molecules move away from the position occupied in the pure reactant crystals. The molecular movements have a rotational component. Additionally, it was found that the adjacent monomer molecules moved closer to each other along with the progress of the phototransformation of crystals.High pressure itself also causes changes in crystal structures, such as shortening of intermolecular distances, modification of molecular orientation and decrease of volume and elasticity of reaction cavities.6–13 It can also reduce structural changes that come about by photochemical reactions and influence reaction kinetics.12
Until now, crystallographic studies on monitoring a course of photochemical reactions at high pressure have been carried out for intramolecular photocyclization reactions, namely, for the formation of a five-membered ring in 2-tert-butylphenylphenylmethanone and a four-membered ring (the Norrish–Yang reaction) in 4-(2,4,6-triisopropylbenzoyl)benzoate benzylammonium and 6,6-diethyl-5-oxo-5,6,7,8-tetrahydronaphthalene-2-carboxylate (1S)-1-(4-methylphenyl)ethylammonium11–13 and for [2 + 2] photodimerization of 2,6-difluorocinnamic acid.14 It is worth adding that Boldyreva and co-workers studied the effect of high pressure on the photochemical nitro–nitrito isomerization on the grounds of analysis of pure component crystalline materials (cobalt(III) nitro- and nitritopentaammoniates) using X-ray diffractometry and IR spectroscopy.10,15,16 In this paper, we have presented the results of the studies from monitoring the structural transformations brought about by the [2 + 2] photodimerization of BBCP at high pressure and the comparison with the respective data for ambient pressure.
Experimental
The crystals of BBCP were recrystallized from toluene. The high pressure studies were carried out for four of them at 0.5, 0.7, 1.0 and 1.2 GPa, respectively. Each crystal was separately placed in a Boehler–Almax17 or a Merrill–Bassett18 high pressure cells (DAC) together with a quartz crystal. The quartz served as a high-pressure sensor: the values of pressure were determined on the grounds of its cell parameters.19 As a hydrostatic liquid, a mixture of glycerin and water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was used. The DAC was mounted on a diffractometer and aligned using the gasket-shadow centering procedure.20 The X-ray data were collected by means of a single-crystal X-ray diffractometer equipped with an EOS CCD detector in the dark. The CrysAlisPro program suite21 was used for the data collection, UB matrices determination and data reduction. Additional corrections of reflection intensities, associated with DAC absorption, were used for the partially reacted crystal structures at 0.5 GPa. In the case of the remaining structures, the absorption corrections did not improve the data.
2) was used. The DAC was mounted on a diffractometer and aligned using the gasket-shadow centering procedure.20 The X-ray data were collected by means of a single-crystal X-ray diffractometer equipped with an EOS CCD detector in the dark. The CrysAlisPro program suite21 was used for the data collection, UB matrices determination and data reduction. Additional corrections of reflection intensities, associated with DAC absorption, were used for the partially reacted crystal structures at 0.5 GPa. In the case of the remaining structures, the absorption corrections did not improve the data.
The photochemical reaction was induced by UV radiation in the dark. The crystals in DAC were irradiated in stages using an Hg 100 W lamp equipped with a water filter and a BG-39 Andover glass filter. The BG-39 glass filter has a maximum transmittance at about 495 nm and a cut-on at about 320 nm. The chosen filter ensured homogeneity of the studied photochemical reaction.22,23 It was a result of our estimates that 99% of UV radiation was absorbed in the crystal layer of 0.12 mm for the wavelength of 325 nm (the wavelength from the low-energy absorption tail). The thickness of this layer was comparable with the thickness of the studied crystals. This means that the distribution of product molecules was almost uniform throughout the crystals. The time of UV irradiation of the studied crystals and the product concentration are given in Table 1. The product concentration was provided by the site occupation factor refined during the X-ray structure analysis. The structures were refined using SHELXL2014.24,25 The initial coordinates for the pure-component crystals were taken from the structure determined under ambient conditions (CCDC 154265; REFCODE: BZDCPB08).3 The phenyl rings for all crystals were refined as rigid rotating groups of geometry also taken from the pure-component structures determined under ambient conditions.3 Most atoms in the partially reacted crystals were refined as disordered atoms over two positions: one for the reactant and the second for the product. Due to the reactant–product disorder, the following weak restraints from SHELXL2014 were applied: DFIX, DANG, FLAT and SIMU. The target values for bond lengths and valence angles were taken from the structures of the pure reactant and pure product crystals under ambient conditions. Due to a small number of observed reflections, most non-hydrogen atoms of reactant and product molecules were refined isotropically. The oxygen atom of the major component was treated anisotropically, except for the crystal structures containing 26.2% and 54.4% of the product at 0.5 GPa. In the case of the pure dimer crystals at 0.5 GPa and 1.0 GPa and for the partially reacted crystals at 0.7 GPa, several atoms in phenyl rings were also refined anisotropically, i.e. C9D → C11D and C16D → C18D for 0.5 GPa, C8D → C12D and C15D → C19D for 1.0 GPa and C7M → C12M and C9M → C11M for the crystals containing 12.1 and 49.3%, respectively, of the product at 0.7 GPa. M and D denote monomer and dimer molecules. For all structures, hydrogen atoms were positioned geometrically with Uiso = 1.2Ueq.
| Crystal number | Pressure [GPa] | UV irradiation time [sec] | Product content [%] |
|---|---|---|---|
| 1 | 0.5 | 0 | 8.8(9) |
| 30 | 26.2(11) | ||
| 60 | 35.4(11) | ||
| 90 | 54.4(11) | ||
| 150 | 80.1(13) | ||
| 390 | 100 | ||
| 2 | 0.7 | 0 | 12.1(7) |
| 60 | 49.3(13) | ||
| 3 | 1.0 | 0 | 49.9(11) |
| 2400 | 100 | ||
| 4 | 1.2 | 0 | 80.4(13) |
| 600 | 100 |
Selected experimental data are presented in Table 2 for all structures.
| 0.5 GPa | ||||||
|---|---|---|---|---|---|---|
| 8.8% P | 26.2% P | 35.4% P | 54.4% P | 80.1% P | 100% P | |
| Chemical formula | C19H18O | C19H18O | C19H18O | C19H18O | C19H18O | C19H18O |
| Formula weight | 262.33 | 262.33 | 262.33 | 262.33 | 262.33 | 262.33 |
| Crystal dimensions (mm) | 0.30 × 0.20 × 0.12 | 0.30 × 0.20 × 0.12 | 0.30 × 0.20 × 0.12 | 0.30 × 0.20 × 0.12 | 0.30 × 0.20 × 0.12 | 0.30 × 0.20 × 0.12 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | Pbca | Pbca | Pbca | Pbca | Pbca | Pbca |
| a (Å) | 31.07(4) | 31.16(6) | 31.05(5) | 31.05(5) | 30.99(3) | 30.83(3) |
| b (Å) | 10.5077(13) | 10.4812(19) | 10.4910(16) | 10.4910(16) | 10.5162(12) | 10.5572(11) |
| c (Å) | 8.5590(9) | 8.5557(14) | 8.5775(13) | 8.5625(13) | 8.5388(9) | 8.5066(9) |
| V (Å3) | 2794(4) | 2794(5) | 2794(5) | 2789(5) | 2783(3) | 2769(3) |
| Z | 8 | 8 | 8 | 8 | 8 | 8 |
| D x (Mg m−3) | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.26 |
| μ (mm−1) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| T (K) | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) |
| Reflections collected | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138 |
9980 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 162 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 131 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099 099 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251 251 |
| Reflections independent | 751 | 688 | 740 | 743 | 751 | 757 |
| Reflections observed | 375 | 313 | 353 | 331 | 372 | 429 |
| Completeness (%) | 32.5 | 29.7 | 32.2 | 32.3 | 32.7 | 33.2 |
| R int | 0.208 | 0.222 | 0.235 | 0.237 | 0.238 | 0.160 |
| R (I > 2σ(I)) wR (all), S | 0.118 0.379, 1.09 | 0.137 0.424, 1.32 | 0.133 0.435, 1.36 | 0.146 0.450, 1.41 | 0.120 0.373, 1.05 | 0.103 0.304, 1.04 |
| Δρmax, Δρmin (e Å−3) | 0.19, −0.15 | 0.16, −0.14 | 0.16, −0.12 | 0.17, −0.14 | 0.22, −0.14 | 0.16, −0.18 |
| 0.7 GPa | 1.0 GPa | 1.2 GPa | ||||
|---|---|---|---|---|---|---|
| 12.1% P | 49.3% P | 49.9% P | 100% P | 80.4% P | 100% P | |
| Chemical formula | C19H18O | C19H18O | C19H18O | C19H18O | C19H18O | C19H18O |
| Formula weight | 262.33 | 262.33 | 262.33 | 262.33 | 262.33 | 262.33 |
| Crystal dimensions (mm) | 0.20 × 0.20 × 0.12 | 0.20 × 0.20 × 0.12 | 0.20 × 0.18 × 0.16 | 0.20 × 0.18 × 0.16 | 0.24 × 0.14 × 0.10 | 0.24 × 0.14 × 0.10 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | Pbca | Pbca | Pbca | Pbca | Pbca | Pbca |
| a (Å) | 30.934(2) | 30.841(2) | 30.88(4) | 30.68(3) | 30.37(3) | 30.46(3) |
| b (Å) | 10.379(3) | 10.374(3) | 10.2558(9) | 10.3153(7) | 10.2318(12) | 10.2338(10) |
| c (Å) | 8.5247(12) | 8.5367(13) | 8.4545(9) | 8.3651(6) | 8.3984(9) | 8.3341(9) |
| V (Å3) | 2737.0(9) | 2731.3(9) | 2678(3) | 2647(3) | 2610(3) | 2598(3) |
| Z | 8 | 8 | 8 | 8 | 8 | 8 |
| D x (Mg m−3) | 1.27 | 1.28 | 1.30 | 1.32 | 1.34 | 1.34 |
| μ (mm−1) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| T (K) | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) |
| Reflections collected | 9630 | 8666 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 256 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508 508 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316 316 |
| Reflections independent | 984 | 964 | 814 | 821 | 877 | 866 |
| Reflections observed | 515 | 451 | 409 | 520 | 404 | 436 |
| Completeness (%) | 38.6 | 37.6 | 30.8 | 31.5 | 34.0 | 33.9 |
| R int | 0.118 | 0.150 | 0.097 | 0.088 | 0.144 | 0.134 |
| R (I > 2σ(I)) wR (all), S | 0.085 0.243, 1.04 | 0.122 0.360, 1.06 | 0.089 0.296, 1.11 | 0.066 0.202, 1.06 | 0.092 0.300, 1.05 | 0.103 0.332, 1.08 |
| Δρmax, Δρmin (e Å−3) | 0.16, −0.17 | 0.18, −0.16 | 0.16, −0.12 | 0.14, −0.15 | 0.22, −0.18 | 0.25, −0.26 |
It is worth mentioning that in order to check the sensitivity of crystals of BBCP to light filtered through room windows, we collected X-ray data for another crystal kept under day-light conditions at ambient pressure for 2 weeks and afterwards for an additional 4 weeks. The X-ray structure analysis revealed that the product content changed from 28.9(4) to 70.8(4)%. This indicated that crystals of BBCP should be stored and experiments should be conducted in the dark.
Results and discussion
It is clear from our studies that the [2 + 2] photodimerization of BBCP proceeds in crystals not only at ambient pressure but also under high-pressure conditions and that it proceeds completely. In order to monitor the path and kinetics of this photochemical reaction, the high-pressure structures of crystals containing both the reactant and the product in various proportions were determined. Such crystals feature a reactant–product disorder. Fig. 1 presents an example of the structure of the partially reacted crystal at high pressure. Knowledge of such structures has allowed us to learn about the behaviour of reactant and product molecules, the kind and extent of structural changes in crystals brought about by the photochemical reaction and about the reaction kinetics. The studies have also provided knowledge of the influence of high pressure on the above-mentioned issues.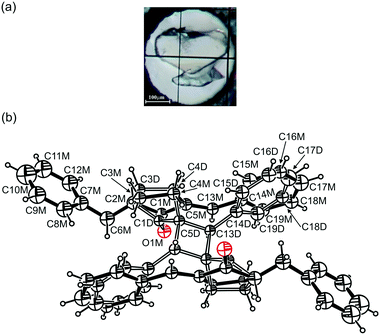 | ||
| Fig. 1 (a) The BBCP (top) and quartz (bottom) crystals at 0.5 GPa. (b) Two monomer molecules superimposed on one dimer molecule in the crystal of 54.4(11)% reaction progress at 0.5 GPa, prepared by means of ORTEP software.26 The dimer molecule lies about an inversion centre (for all crystal structures). Atomic displacement parameters are drawn at a 20% probability level. | ||
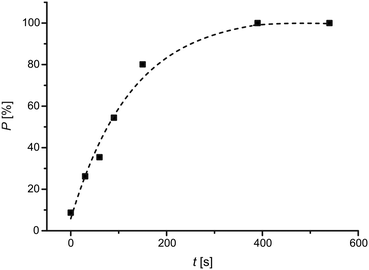
The [2 + 2] photodimerization of BBCP at high pressure is of the first order and proceeds quicker at the beginning than in the final stages. The relationship evidencing this fact, namely the relationship between the percentage content of the product and the time of crystal irradiation, is presented in Fig. 2. The character of this relationship is similar to the one described in the scientific literature for ambient conditions.3 The analysis of the JMAK kinetic equation27–30 confirmed that the [2 + 2] photodimerization of BBCP proceeds in a homogeneous manner at high pressure. The Avrami exponent27–30 for the reaction at 0.5 GPa is 1.04(18), which indicates that the formation of the product proceeds with homogenous dispersion. The homogeneity of the [2 + 2] photodimerization of BBCP in crystals was also observed in the case under ambient conditions (see the Introduction section). The JMAK model is widely used as a description of kinetics of photochemical reactions in crystals.31–36
The reaction rate is greater at 0.7 GPa than at 0.5 GPa. At 0.5 GPa, the product content in the crystal changed from 8.8(9) to 35.4(11)% after 60 s of UV irradiation. At 0.7 GPa, the product content changed more in the same time, namely from 12.1(7) to 49.3(13)%. The difference in the reactivity is not very high, but statistically significant. The comparison of the reaction rate at 0.5 GPa with the relevant literature data for 0.1 MPa cannot be carried out because of slightly different crystal dimensions and experimental conditions applied in the past at 0.1 MPa.3 Nevertheless, the comparison between the structural changes at both pressures can be made. The increase of photochemical reactivity along with high pressure was also observed in the case of 2,6-difluorocinnamic acid.14
What are the reasons for the [2 + 2] photodimerization of BBCP proceeding with a greater rate at 0.7 GPa than at 0.5 GPa? The following factors should be considered in order to address this question, namely: the distance between adjacent monomer molecules, the volume of the unit cell, the volume of free space in crystals and the force of intermolecular interactions.
In general, the shorter the distance between reacting atoms, the quicker the [2 + 2] photodimerization should be. Applying high pressure to crystals causes intermolecular distances to become shorter. However, it is possible that for several distances this will not be the case (for instance when molecules will change their mutual orientation). Such a situation takes place for the BBCP crystals at 0.5 and 0.7 GPa, for which the distance between reactive carbon atoms in adjacent monomer molecule, D, is almost constant: 3.962(13) and 3.954(13) Å for the crystal containing 8.8(9)% of the product at 0.5 GPa and the crystal containing 12.1(7)% of the product at 0.7 GPa, respectively, and also 3.80(3) and 3.82(3) Å for the crystal containing 54.4(11)% of the product at 0.5 GPa and the crystal containing 49.3(13)% of the product at 0.7 GPa, respectively. This clearly indicates that the distance between reactive carbon atoms is not the reason for the higher reactivity of BBCP at 0.7 GPa. It should be emphasized that the constancy of D at 0.5 and 0.7 GPa exists despite the decrease in the cell volume and all cell parameters at 0.7 GPa.
The next factor which should be taken into account is connected with the cell volume. In the case of BBCP studied at ambient pressure, it was observed that the cell volume decreased along with the reaction progress; however, that decrease was not very high.3 In general, consistency between the direction of structural changes brought about by a reaction and by high pressure should make the reaction easier.7,8,12 This means that due to the decrease in the cell volume with the reaction progress, the photochemical reaction of BBCP should be faster at higher pressure.
Nevertheless, other factors exist which could counteract the influence of the aforementioned factor. One of them is the volume of free space, which should be taken into account not only before but also during the whole phototransformation. Such a volume for the crystals with a 50% reaction progress is presented in Fig. 3. As can be seen, the free space in the crystal and also in the region of two reacting monomer molecules (marked by the dashed line) decreases en route from 0.1 MPa to 0.7 GPa. A decrease of free space usually makes a reaction more difficult. However, in the case of BBCP, the dimer molecules fit very well to the lattice of the monomer molecules and do not demand additional space (see Fig. 1). This suggests that the observed decrease in free space is not crucial for the BBCP reactivity.
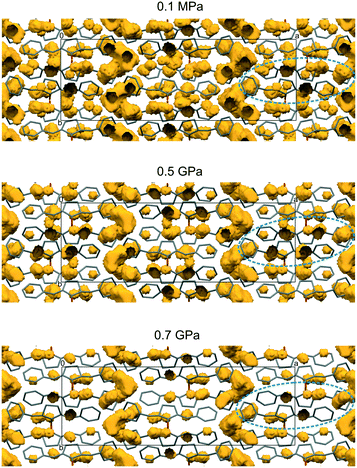 | ||
| Fig. 3 The crystal lattice for the following reaction progress: 55.6(4)%, 54.4(11)% and 49.3(13)% at 0.1 MPa, 0.5 GPa and 0.7 GPa, respectively. The free space is shown in yellow. The dashed line indicates one pair of the monomer molecules which will create the dimer. The product molecules were omitted. The figure was prepared with the Mercury CSD 3.8 program.37 The radius of the rolling ball was 0.6 Å and the grid was 0.2 Å. The part of the figure concerning 0.1 MPa was prepared on the grounds of data taken from ref. 3 (CCDC 154258; REFCODE: BZDCPB01). | ||
The strong intermolecular interactions are the next factor which could influence the rate of the [2 + 2] photodimerization of BBCP at high pressure. The intermolecular interactions of the compound were visualized by the Hirshfeld surfaces. Fig. 4. presents such surfaces for the crystal with about a 50% reaction progress at 0.1 MPa, 0.5 GPa and 0.7 GPa and the geometry of close intermolecular interactions is given in Table 3. Interestingly, four red spots seen at 0.5 GPa on the Hirshfeld surface and connected with the C–H⋯C interactions with the partnering monomer molecule disappeared at 0.7 GPa (despite the smaller cell parameters and the cell volume at 0.7 GPa). This signifies that the mutual arrangement of the monomer molecules is slightly different at both pressures. It can also be seen in Fig. 4 that at 0.7 GPa there are shorter C–H⋯C interactions with other surrounding molecules. Since all the above-mentioned contacts are rather far from the reaction centre, they should not have a significant influence on atomic shifts in the reaction centre and on the BBCP reactivity. (The red spot which appeared at atom C4M at 0.7 GPa stands for a weak H⋯H interaction.)
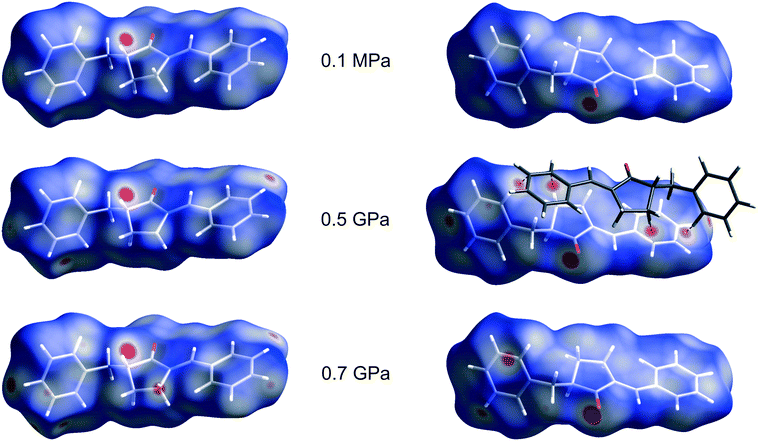 | ||
| Fig. 4 The Hirshfeld surface of the BBCP monomer for 55.6(4)% reaction progress at 0.1 MPa, 54.4(11)% reaction progress at 0.5 GPa, and 49.3(13)% reaction progress at 0.7 GPa seen from two opposite sides. The surfaces were calculated and visualized by means of the Crystal Explorer (v. 3.1) program.38,39 The red spots stand for intermolecular contacts shorter than the sum of the van der Waals radii and the dashed lines indicate contacts between the molecule inside the Hirshfeld surface (white) and the monomer molecule which will react with it (dark grey). | ||
| D–H [Å] | H⋯A [Å] | D⋯A [Å] | D–H⋯A [°] | |
|---|---|---|---|---|
| Symmetry codes: i = x, 1.5 − y, 0.5 + z; ii = 1 − x, 1 − y, −z; iii = 0.5 − x, 1 − y, 0.5 + z; iv = 0.5 + x, y, 0.5 − z; v = 1 − x, 0.5 + y, 0.5 − z; vi = 1 − x, 1 − y, 1 − z, vii = 0.5 − x, −0.5 + y, z, viii = x, 1.5 − y, −0.5 + z. | ||||
| 0.1 MPa | ||||
| C2M–H2M⋯O1Mi | 0.98 | 2.45 | 3.286(4) | 143.3 |
| 0.5 GPa | ||||
| C2M–H2M⋯O1Mi | 0.98 | 2.38 | 3.215(17) | 142.6 |
| C3M–H3M1⋯C19Mii | 0.97 | 2.70 | 3.65(7) | 167.2 |
| C8M–H8M⋯C18Mii | 0.95 | 2.68 | 3.57(11) | 154.6 |
| C10M–H10M⋯C8Miii | 0.95 | 2.84 | 3.74(4) | 159.4 |
| C17M–H17M⋯C10Miv | 0.95 | 2.75 | 3.47(6) | 132.8 |
| C18M–H18M⋯C9Mv | 0.95 | 2.75 | 3.41(9) | 128.1 |
| 0.7 GPa | ||||
| C2M–H2M⋯O1Mi | 0.98 | 2.35 | 3.185(14) | 142.6 |
| C10M–H10M⋯C8Miii | 0.95 | 2.71 | 3.62(2) | 162.8 |
| C18M–H18M⋯C9Mv | 0.94 | 2.79 | 3.49(8) | 133.0 |
| C17M–H17M⋯C10Mvi | 0.95 | 2.84 | 3.59(4) | 137.6 |
| C9M–H9M⋯C11Mvii | 0.94 | 2.78 | 3.66(3) | 155.3 |
| C6M–H6M1⋯C12Mviii | 0.97 | 2.80 | 3.55(3) | 134.0 |
Taking into account the above considerations, it can be said that the decrease in the cell volume with the reaction progress is the reason for the increase in the reaction rate at 0.7 GPa and that the D distance between reactive atoms, the volume of free space and the intermolecular interactions are not the factors which bring about this increase.
From the above-given values of the D distance at 0.5 and 0.7 GPa, it is seen that D decreases during the photochemical transformation of BBCP crystals. We might ponder the question: is this trend valid for the whole process? In the case of the [2 + 2] photodimerization of BBCP and the derivative of BBCP (2-benzylidene-5-(4-chlorobenzyl)cyclopentanone, BBCPCl) under ambient conditions, the distance between reactive carbon atoms in adjacent monomer molecules decreased during the whole crystal phototransformation.3,40,41 That relationship was explained by the increase of stress of product molecules imposed on reactant molecules: the more the product, the stronger the effect. Fig. 5 presents the relationship between the D distance and the content of the product for BBCP at 0.5 GPa in comparison to the relevant data for 0.1 MPa. In the case of high pressure, the decrease is observed only during the first part of the phototransformation and it is statistically significant at the 3σ level. In comparison to ambient conditions, the slope of the line fitted to the high-pressure data is bigger, which indicates that the stress induced by product molecules on monomer pairs is more effective at high pressure. After the observed decrease, the D distance becomes statistically constant at the 3σ level, which shows a certain limit of effectiveness of stress imposed by product molecules on monomer pairs resulting from closer intermolecular distances between monomer molecules at 0.5 GPa. The decrease of the D distance was also noticed for the first part of the photochemical transformation in the crystals of 2,6-difluorocinnamic acid at high pressure.14
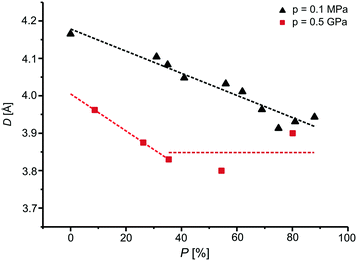 | ||
| Fig. 5 The relationship between the distance between reactive carbon atoms and the content of the product in the crystal of BBCP at 0.1 MPa and 0.5 GPa. The part of the figure concerning 0.1 MPa was prepared on the grounds of data taken from ref. 3. | ||
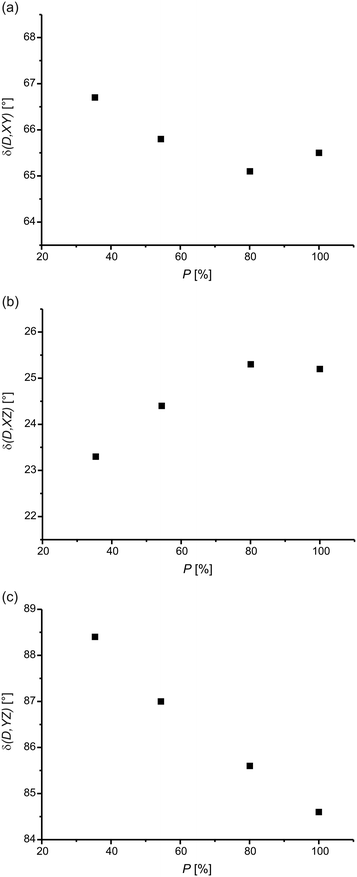
The [2 + 2] photodimerization of BBCP at high pressure causes changes in the orientation of molecules and molecular fragments in the unit cell. Fig. 6 shows the changes in the orientation of the dimer molecule along with the reaction progress at 0.5 GPa. The comparison of these relationships with the relevant data for ambient pressure reveals that the direction and extent of the changes are very similar at both pressures. As can also be seen, the structural changes are smooth at high pressure. This was the same for ambient conditions.3
The positions of atoms change along with the progress of the photochemical reaction, the consequence of which is the changes in the cell parameters. The variations in the cell volume of BBCP for different values of pressure are shown in Fig. 7. The cell volume does not change in the first part of the crystal phototransformation, but alters slightly in the remaining part, which is connected with the similar shape of the dimer and one pair of the monomers. The range of the changes in the cell volume caused by the [2 + 2] photodimerization is quite similar for ambient and high pressures.
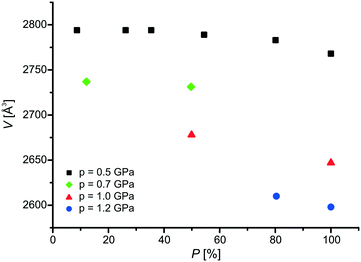 | ||
| Fig. 7 The variations in the cell volume along with the percentage content of the product for 0.5 GPa, 0.7GPa, 1.0 GPa and 1.2 GPa. | ||
High pressure itself (without the photochemical reaction) influences the weak geometrical parameters such as intermolecular contacts and intramolecular torsion angles, but it does not influence the hard geometrical parameters such as bond lengths and valence angles of BBCP. From Fig. 8 it is seen that the shape of BBCP molecules only depends slightly on the pressure applied. The same was observed for other compounds studied at high pressure.11,12,14
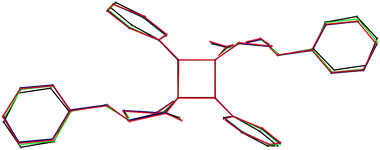 | ||
| Fig. 8 The superposition of the dimer molecules for 0.1 MPa (black), 0.5 GPa (green), 1.0 GPa (blue) and 1.2 GPa (red). Hydrogen atoms are omitted for clarity. The part of the figure concerning 0.1 MPa was prepared on the grounds of the data taken from ref. 3. | ||
Conclusions
We presented the results on monitoring the structural changes that came about by the [2 + 2] photodimerization in crystals of BBCP at high pressure. This is one of the two first examples of such studies for an intermolecular reaction. On the grounds of the comparison of the high pressure structural data with the relevant data under ambient conditions, we can say that the influence of pressure of 0.5 GPa on (a) the extent of the changes in the cell volume caused by the photochemical reaction, (b) the extent of variations in molecular orientation during the crystal phototransformation and (c) the decrease of the D distance between the reactive atoms brought about by the stress of product molecules are the same or very similar. However, the character of the changes in the D distance is different for 0.1 MPa and 0.5 GPa. The reason for the observed close similarity between the reaction path at both pressures is a very good fit for dimer molecules to the crystal lattice of the monomer. We also discovered that the photochemical reactivity of BBCP is greater at 0.7 GPa than 0.5 GPa (however, the difference is not very significant) and explained this fact by the consistency between structural changes caused by the photochemical reaction and by high pressure.Acknowledgements
This work was financed by the statutory activity subsidy of the Polish Ministry of Science and Higher Education.References
- H. Nakanishi, W. Jones, J. M. Thomas, M. B. Hursthouse and J. M. Motevalli, J. Phys. Chem., 1981, 85, 3636–3642 CrossRef CAS.
- K. Honda, F. Nakanishi and N. Feeder, J. Am. Chem. Soc., 1999, 121, 8246–8250 CrossRef CAS.
- I. Turowska-Tyrk, Chem. – Eur. J., 2001, 7, 3401–3405 CrossRef CAS.
- I. Turowska-Tyrk, Chem. Phys. Lett., 2002, 361, 115–120 CrossRef CAS.
- G. Kaupp, Curr. Opin. Solid State Mater. Sci., 2002, 6, 131–138 CrossRef CAS.
- E. V. Boldyreva, Solid State Ionics, 1997, 101–103, 843–849 CrossRef CAS.
- E. V. Boldyreva, S. L. Kuzmina and H. Ahsbahs, J. Struct. Chem., 1998, 39, 762–773 CrossRef CAS.
- E. V. Boldyreva, Russ. J. Coord. Chem., 2001, 27, 297–323 CrossRef CAS.
- M. Tapilin, N. N. Bulgakov, A. P. Chupakhin, A. A. Politov and A. G. Druganov, J. Struct. Chem., 2010, 51, 635–641 CrossRef.
- B. A. Zakharov, A. S. Marchuk and E. V. Boldyreva, CrystEngComm, 2015, 17, 8812–8816 RSC.
- J. Bąkowicz and I. Turowska-Tyrk, CrystEngComm, 2014, 16, 6039–6048 RSC.
- K. Konieczny, J. Bąkowicz and I. Turowska-Tyrk, CrystEngComm, 2015, 17, 7693–7701 RSC.
- K. Konieczny, J. Bąkowicz and I. Turowska-Tyrk, J. Photochem. Photobiol., A, 2016, 325, 111–115 CrossRef CAS.
- T. Galica, J. Bąkowicz, K. Konieczny and I. Turowska-Tyrk, CrystEngComm, 2016 10.1039/c6ce01652a.
- E. V. Boldyreva, S. L. Kuzmina and H. Ahsbahs, J. Struct. Chem., 1998, 39, 343–349 CrossRef CAS.
- E. V. Boldyreva, D. Yu. Naumov and H. Ahsbahs, J. Struct. Chem., 1998, 39, 350–361 CrossRef CAS.
- R. Boehler, Rev. Sci. Instrum., 2006, 77, 115103 CrossRef.
- L. Merrill and W. A. Bassett, Rev. Sci. Instrum., 1974, 45, 290–294 CrossRef.
- R. J. Angel, D. R. Allan, R. Miletich and W. Finger, J. Appl. Crystallogr., 1997, 30, 461–466 CrossRef CAS.
- A. Budzianowski and A. Katrusiak in High-Pressure Crystallography, ed. A. Katrusiak and P. F. McMillan, Kluwer Academic Publishers, Dordrecht Boston London, 2004, pp. 101–112 Search PubMed.
- Rigaku Oxford Diffraction, CrysAlis PRO., Rigaku Oxford Diffraction, Wrocław, Poland, 2015 Search PubMed.
- V. Enkelmann, G. Wegner, K. Novak and K. B. Wagener, J. Am. Chem. Soc., 1993, 115, 10390–10391 CrossRef CAS.
- K. Novak, V. Enkelmann, G. Wegner and K. B. Wagener, Angew. Chem., Int. Ed. Engl., 1993, 32, 1614–1616 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- M. Avrami, J. Chem. Phys., 1939, 7, 1103–1112 CrossRef CAS.
- W. A. Johnson and R. F. Mehl, Trans. Am. Inst. Min. Metall. Eng., 1939, 135, 416–458 Search PubMed.
- A. N. Kolmogorov, Bull. Acad. Sci. USSR, Math. Ser., 1937, 1, 355–359 Search PubMed.
- J. Bąkowicz, J. Olejarz and I. Turowska-Tyrk, J. Photochem. Photobiol., A, 2014, 273, 34–42 CrossRef.
- N. K. Nath, T. Runčevski, C.-Y. Lai, M. Chiesa, R. E. Dinnebier and P. Naumov, J. Am. Chem. Soc., 2015, 137, 13866–13875 CrossRef CAS PubMed.
- T. V. Sreevidya, D.-K. Cao, T. Lavy, M. Botoshansky and M. Kaftory, Cryst. Growth Des., 2013, 13, 936–941 CAS.
- A. G. Jarvis, H. A. Sparkes, S. E. Tallentire, L. E. Hatcher, M. R. Warren, P. R. Raithby, D. R. Allan, A. C. Whitwood, M. C. R. Cockett, S. B. Duckett, J. L. Clark and I. J. S. Fairlamb, CrystEngComm, 2012, 14, 5564–5571 RSC.
- M. Bertmer, R. C. Nieuwendaal, A. B. Barnes and S. E. Hayes, J. Phys. Chem. B, 2006, 110, 6270–6273 CrossRef CAS PubMed.
- R. Moré, G. Busse, J. Hallmann, C. Paulmann, M. Scholz and S. Techert, J. Phys. Chem. C, 2010, 114, 4142–4148 Search PubMed.
- J. B. Benedict and P. Coppens, J. Phys. Chem. A, 2009, 113, 3116–3120 CrossRef CAS PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer (Version 3.0), University of Western Australia, 2012 Search PubMed.
- I. Turowska-Tyrk, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 670–675 Search PubMed.
- I. Turowska-Tyrk, J. Phys. Org. Chem., 2004, 17, 837–847 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1491722-1491731, 1496289-1496290. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce01650b |
| This journal is © The Royal Society of Chemistry 2016 |