Evaporation-driven regularization of crystallographically ordered arrangements of truncated nanoblocks: from 1D chains to 2D rhombic superlattices
Riho
Matsumoto
,
Yoshitaka
Nakagawa
,
Hiroyuki
Kageyama
,
Yuya
Oaki
and
Hiroaki
Imai
*
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1, Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: hiroaki@applc.keio.ac.jp
First published on 1st August 2016
Abstract
Rhombic superlattices of truncated Mn3O4 nanoblocks were constructed by evaporation-driven assembly. The tetragonal crystal lattice of Mn3O4 in the nanoblocks was clearly shown to be ordered in the superlattices. Tightly packed 2D rhombic superlattices were produced via loosely packed parallel arrays by stacking 1D chains of the nanoblocks elongated in the a direction of the tetragonal lattice by tuning the evaporation rate.
Nanometric building blocks, such as nanospheres, nanorods, and nanoplates, are utilized for a bottom-up approach to achieve sophisticated architectures.1–9 Highly ordered assemblies of uniform nanoblocks called superlattices are built through self-assembly of the building blocks in liquid media.1–4,10–26 By using spherical building blocks, however, the crystallographic direction is not controlled in the ordered arrays. On the other hand, cube- and cuboid-shaped building blocks tend to assemble in the same crystallographic direction, like a single crystal, by facing well-defined facets.15–26 Micrometer- to millimeter-scale ordered arrays of rectangular nanoblocks have been fabricated on a substrate by convective self-assembly.26–30 The assembly of the metal oxide nanoblocks by directing the crystallographic orientation is significant for the practical use of functional materials in various technological applications.22,26,28–30
Controlled arrangements of rectangular nanoblocks are essential for the fabrication of various ordered architectures. Recently, diversely shaped 1D, 2D, and 3D ordered arrays were fabricated via the assembly of anisotropic rectangular Mn3O4 nanoblocks by controlling their crystallographic orientation.29 1D and two kinds of 2D arrays were selectively produced by TiO2 rectangular nanoblocks with a variation of the concentration.31,32 The regularity of ordered arrays of iron oxide nanocubes was controlled by the interaction of trench walls on a substrate.33 A wide variety of assembled gold nanobipyramids were produced at the air–liquid interface by changing the evaporation rate.34 However, further establishment of the controllability of the arrangements is required for the development of ordered architectures via a bottom-up approach.
Well-ordered 2D and 3D arrays of fine particles were spontaneously produced on a solid substrate by evaporation of a colloidal dispersion. Colloidal ordering is regulated by changing various conditions.4 Generally, the regularity of ordered arrays of small building blocks is influenced by the rate of assembly. In the present work, a variety of architectures of direction-controlled anisotropic nanocuboids are achieved via an evaporation-driven assembly.29,31,32 Here, we fabricated elaborate 2D architectures by the crystallographically ordered arrangement of truncated Mn3O4 nanoblocks. The tetragonal crystal lattice in the nanoblocks was ordered in the superlattices. Tightly packed rhombic superlattices and loosely packed parallel arrays were selectively produced by the lateral stacking of 1D chains of the nanoblocks by tuning the evaporation rate. The regularity of the superlattice of the building blocks improved with a decreasing assembly rate. The 2D assembly at the air–liquid interface would be important for crystallographic control of the ordered arrays.
Truncated Mn3O4 nanoblocks as a building block were prepared through a two-phase solvothermal method according to our previous work.29 We dissolved 0.60 mmol manganese(II) chloride and 35 wt% hydrogen peroxide (4 cm3) in 31 cm3 of water in a 100 cm3 Teflon container. Oleic acid (3.97 mmol) and tert-butylamine (2.31 mmol) were added to 30 cm3 of toluene. The organic mixture was added to the Teflon container without stirring. At this time, oxygen gas was generated through the decomposition of hydrogen peroxide. When the generation of oxygen gas stopped, the Teflon container was put into a stainless steel autoclave. The autoclave was heated at 115 °C for 12 h. After the reaction, the resultant dark brown liquid (upper phase) was transferred into a glass vial. A copper grid covered with a collodion film was placed on a piece of filter paper. A drop of the resultant dispersion was placed on the grid. The excess medium of the dispersion was absorbed by the filter paper. The products deposited on the grid were characterized by the transmission electron microscopy (TEM), high-resolution TEM (HRTEM), and fast Fourier transform (FFT) profiles.
Fig. 1 shows truncated Mn3O4 nanocrystals as a building block. The FFT spots corresponding to the lattice fringes of the nanocrystals were assigned to the tetragonal crystal lattice of Mn3O4 (a = 0.576 nm and c = 0.944 nm). The Mn3O4 nanocrystals exhibited truncated cuboids with four {100} faces and two (001) faces. The bezels of the truncated blocks were assigned to {101}. The width and length of the nanocuboids were ca. 10 nm and ca. 20 nm, respectively. The interparticle distance 3.2 nm in width was close to twice the molecular length of oleic acid (1.7 nm). This suggests that the nanoblocks were covered with the molecules that were slightly tilted. The hydrophobic nanoblocks covered with oleic acid molecules were well dispersed in a low polar dispersion medium, such as toluene. As reported in our previous work,29,35 the nanoblocks were easily aligned in the a (<100>) direction on a polymer film of a copper grid (Fig. 1d). Relatively large a faces were attached to each other to effectively reduce the surface energy.
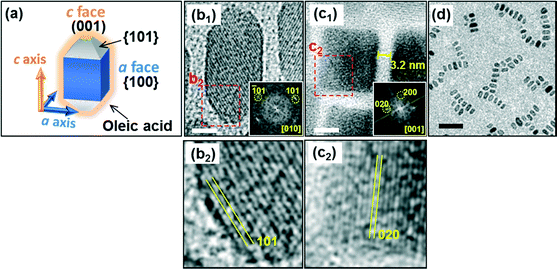 | ||
| Fig. 1 A schematic illustration (a), HRTEM images (b1,2, c1,2), and corresponding FFT patterns (insets) of rectangular Mn3O4 nanoblocks. (b2, c2) enlarged HRTEM images of red squares in (b1) and (c1). (d) TEM image of 1D chains of the nanoblocks on a polymer film of a copper grid. The distance between the nanocuboids indicates the presence of oleic acid. Scale bars: (b1, c1) 5 nm and (d) 50 nm. 1D chains (d) were frequently observed on the copper grid. We occasionally obtained 2D clusters in which the c axis is perpendicular to the surface (c1). Detailed conditions were reported in our previous article.29 | ||
The resultant dispersion of Mn3O4 nanoblocks was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 5 min. The precipitates of 2.2 × 10−3 or 2.8 × 10−1 g dm−3 were redispersed into a hexane–toluene mixture (1
500 rpm for 5 min. The precipitates of 2.2 × 10−3 or 2.8 × 10−1 g dm−3 were redispersed into a hexane–toluene mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) in a 6 cm3 vial by ultrasonication for 30 min. A silicon substrate (7 mm × 16 mm) treated by acetone with ultrasonication for 30 min was put into a 0.5 cm3 dispersion in the vial. The dispersion spread on the substrate by its surface tension. We produced the ordered arrays by evaporation of liquid media in the vial with a cover. The evaporation rate was controlled by changing temperature (7 °C and room temperature) and aperture size of the vial. We calculated the evaporation rate form the evaporated volume of the liquid media during a certain period. When the drying was completed, the morphologies of products were observed via scanning electron microscopy (SEM). We observed the crystalline lattice of the nanoblocks on a copper grid placed on the silicon substrate with HRTEM.
1 in volume) in a 6 cm3 vial by ultrasonication for 30 min. A silicon substrate (7 mm × 16 mm) treated by acetone with ultrasonication for 30 min was put into a 0.5 cm3 dispersion in the vial. The dispersion spread on the substrate by its surface tension. We produced the ordered arrays by evaporation of liquid media in the vial with a cover. The evaporation rate was controlled by changing temperature (7 °C and room temperature) and aperture size of the vial. We calculated the evaporation rate form the evaporated volume of the liquid media during a certain period. When the drying was completed, the morphologies of products were observed via scanning electron microscopy (SEM). We observed the crystalline lattice of the nanoblocks on a copper grid placed on the silicon substrate with HRTEM.
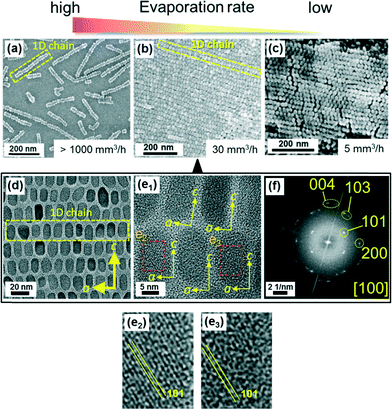
As shown in Fig. 2a, we observed 1D chains of the nanocuboids elongated in the a direction on the substrate after evaporation of the dispersion medium. The chains scattered on the substrate at an extremely high evaporation rate (>1000 mm3 h−1). The 2D superlattices were formed on the substrate through parallel assembly of the 1D chains by decreasing the evaporation rate to 30 mm3 h−1 (Fig. 2b). Fig. 2d shows a TEM image of the 2D superlattice of the nanoblocks that were obtained on a copper grid at the evaporation rate of 30 mm3 h−1. According to the alignment of the fringes in HRTEM images (Fig. 2e) and the presence of spots assigned to Mn3O4 in the FFT image (Fig. 2f), the tetragonal crystal lattice was aligned over the truncated grains in the 2D arrays although the organic layers separated the building blocks. The c and a axes of the tetragonal crystal lattice were basically arranged perpendicular and parallel to the long axis of the 1D chains in the 2D superlattice, respectively. This means that the 1D chains elongated in the a direction of the Mn3O4 crystal were stacked in the c direction on the substrate. A decrease in the evaporation rate by decreasing the ambient temperature produced a 2D array with a tightly packed rhombic superlattice (Fig. 2c). The assembly induced by a gentle evaporation yielded highly ordered 2D arrangements of the truncated nanoblocks. The particle concentration must be larger than a certain value to obtain densely packed arrays on the substrate as shown in Fig. 2. Oleic acid covering nanoblocks is required for stable dispersion of the nanoblocks in organic media. Similarly ordered 2D arrays spread in an area of several square millimeters on the substrate at a certain evaporation rate. The interparticle spacing was basically uniform in the area. However, a domain in which the 1D chains were strictly arranged in the same direction was restricted in an area of several square micrometers.
Quantitative evaluation of the order in the 2D superlattices was performed on the basis of SEM images of the nanoblocks (Fig. 3a) and corresponding FFT patterns (Fig. 3b). Spots assigned to reciprocal lattice vectors 10, 01, and 11 in the reciprocal lattice space indicate the 2D superlattices of nanoblocks in real space (Fig. 3c). According to the FFT patterns, rhombic lattices are basically formed by the assembly of the nanoblocks (Fig. 3c). We obtained the lattice angle θ and the lengths of the a1 and a2 axes of 2D units from 10 and 01 spots in the FFT patterns (Fig. 3d). Since θ was scattered from 65° to 82°, loosely packed parallel arrays were produced by stacking 1D chains of the nanocuboids at a high evaporation rate (Fig. 3c, right). On the other hand, θ closed to 60° with a decrease in the evaporation rate. The length of a1 axis was not drastically changed, while that of a2 axis was slightly increased with the decreasing evaporation rate. The 11 spot was defined in the FFT pattern of the ordered lattices prepared at a relatively low evaporation rate (5 mm3 h−1). These results mean that a tightly packed rhombic lattice was finally produced by decreasing the evaporation rate (Fig. 3c, left). The order of the nanoblocks was improved in the rhombic superlattice by slowing down the assembly rate.
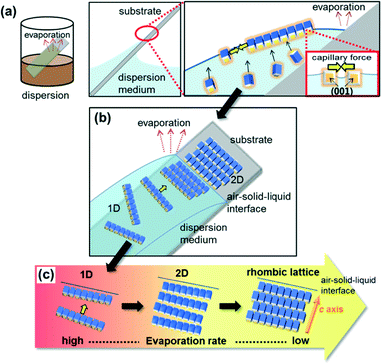
As illustrated in Fig. 4, the ordered arrays of truncated nanoblocks are fabricated on a substrate by convective self-assembly through evaporation of a liquid media from a dispersion. The nanoblocks covered with oleic acid are concentrated at the air-liquid interface due to their hydrophobicity (Fig. 4a). As the interface shrinks during evaporation, the 1D chains of the nanoblocks are formed in the a direction through the attachment of relatively large a faces by a lateral capillary force. The 1D chains are stacked in the c direction at the air–solid–liquid interface as the dispersion recedes on the substrate with evaporation (Fig. 4b). The parallel arrays are roughly ordered when the 1D chains are stacked rapidly. Tightly packed 2D rhombic superlattices are constructed with gentle assembly of the 1D chains by decreasing the evaporation rate (Fig. 4c).
Basically, ordered 2D arrays are produced as a monolayer of the nanoblocks at the air–liquid interface as shown in Fig. 4. However, some clusters of the nanoblocks are frequently formed and precipitate on the substrate when the evaporation proceeds very slowly. We then observed nanoblocks under the ordered 2D superlattices.
In the present study, Mn3O4 was used a typical example of anisotropic building blocks because we can produce truncated nanoblocks of Mn3O4 having the same size and shape by a simple method. Strict control of the block size and shape is essential for the ordered assembly. Since the blocks are covered with oleic acid, the chemical composition is not important for the assembly. In consequence, the current work provides a hint for a general method to produce highly ordered arrays of a wide variety of functional nanomaterials. For example, the ordered arrays of manganese oxides are applicable for highly functional catalysts and electrode materials exposing specific crystal surfaces. In our previous work, we have reported that the ordered arrays of Mn3O4 incorporated with polypyrrole exhibited a high capacity as an anode of lithium-ion battery.30
In summary, we fabricated various types of ordered 2D superarchitectures consisting of truncated Mn3O4 nanoblocks by tuning the assembly rate. The anisotropic shape of the building blocks is essential for controlling the orientation of the crystal lattice in the superlattices. The assembly of building blocks is influenced by their shape and size but not by their chemical composition. Therefore, the evaporation-driven regularization of the crystallographically ordered superlattices of anisotropic nanoscale building blocks is regarded as a novel direction-controlled accumulation technique for the bottom-up fabrication of advanced materials.
Acknowledgements
This work was partially supported by Grant-in-Aid for Challenging Exploratory Research (15K14129) and Grant-in-Aid for Scientific Research (A) (16H02398) from Japan Society for the Promotion of Science.References
- F. X. Redl, K.-S. Cho, C. B. Murray and S. O'Brien, Nature, 2003, 423, 968 CrossRef CAS PubMed.
- E. V. Shevchenko, D. V. Talapin, C. B. Murray and S. O'Brien, J. Am. Chem. Soc., 2006, 128, 3620 CrossRef CAS PubMed.
- W. H. Evers, H. Friedrich, L. Filion, M. Dijkstra and D. Vanmaekelbergh, Angew. Chem., Int. Ed., 2009, 48, 9655 CrossRef CAS PubMed.
- D. T. W. Toolan, S. Fujii, S. J. Ebbens, Y. Nakamura and J. R. Howse, Soft Matter, 2014, 10, 8804 CAS.
- B. T. Diroll, N. J. Greybush, C. R. Kagan and C. B. Murray, Chem. Mater., 2015, 27, 2998 CrossRef.
- X. Ye, J. A. Millan, M. Engel, J. Chen, B. T. Diroll, S. C. Glotzer and C. B. Murray, Nano Lett., 2013, 13, 4980 CrossRef CAS PubMed.
- T. Paik and C. B. Murray, Nano Lett., 2013, 13, 2952 CrossRef CAS PubMed.
- A. E. Saunders, A. Ghezelbash, D.-M. Smilgies, M. B. Sigman Jr. and B. A. Korgel, Nano Lett., 2006, 6, 2959 CrossRef CAS PubMed.
- K. J. Si, D. Sikdar, Y. Chen, F. Eftekhari, Z. Xu, Y. Tang, W. Xiong, P. Guo, S. Zhang, Y. Lu, Q. Bao, W. Zhu, M. Premaratne and W. Cheng, ACS Nano, 2014, 8, 11086 CrossRef CAS PubMed.
- T. P. Bigioni, X.-M. Lin, T. T. Nguyen, E. I. Corwin, T. A. Witten and H. M. Jaeger, Nat. Mater., 2006, 5, 265 CrossRef CAS PubMed.
- H. Yang, T. Ogawa, D. Hasegawa and M. Takahashi, J. Appl. Phys., 2008, 103, 07D526 Search PubMed.
- Y. Wang, P. Kanjanaboos, S. P. McBride, E. Barry, X.-M. Lin and H. M. Jaeger, Faraday Discuss., 2015, 181, 325 RSC.
- W. Han and Z. Lin, Angew. Chem., Int. Ed., 2012, 51, 1534 CrossRef CAS PubMed.
- A. Fortini and M. Dijkstra, J. Phys.: Condens. Matter, 2006, 18, L371 CrossRef CAS PubMed.
- F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Cryst. Growth Des., 2010, 10, 4537 CAS.
- J. Zhang, H. Yang, K. Yang, J. Fang, S. Zou, Z. Luo, H. Wang, I.-T. Bae and D. Y. Jung, Adv. Funct. Mater., 2010, 20, 3727 CrossRef CAS.
- A. Demortie're, P. Launois, N. Goubet, P.-A. Albouy and C. Petit, J. Phys. Chem. B, 2008, 112, 14583 CrossRef PubMed.
- F. Dang, K. Mimura, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Nanoscale, 2012, 4, 1344 RSC.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131 CrossRef CAS PubMed.
- X. S. Shen, G. Z. Wang, X. Hong and W. Zhu, CrystEngComm, 2009, 11, 753 RSC.
- T. Wang, X. Wang, D. LaMontagne, Z. Wang, Z. Wang and Y. C. Cao, J. Am. Chem. Soc., 2012, 134, 18225 CrossRef CAS PubMed.
- C.-J. Chen, R.-K. Chiang and Y.-R. Jeng, J. Phys. Chem. C, 2011, 115, 18142 CAS.
- D. Yu and V. W.-W. Yam, J. Am. Chem. Soc., 2004, 126, 13200 CrossRef CAS PubMed.
- A. P. LaGrow, B. Ingham, S. Cheong, G. V. M. Williams, C. Dotzler, M. F. Toney, D. A. Jefferson, E. C. Corbos, P. T. Bishop, J. Cookson and R. D. Tilley, J. Am. Chem. Soc., 2012, 134, 855 CrossRef CAS PubMed.
- F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Cryst. Growth Des., 2011, 11, 4129 CAS.
- K. Mimura, F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Jpn. J. Appl. Phys., 2011, 50, 09NC09 CrossRef.
- J.-M. Meijer, F. Hagemans, L. Rossi, D. V. Byelov, S. I. R. Castillo, A. Snigirev, I. Snigireva, A. P. Philipse and A. V. Petukhov, Langmuir, 2012, 28, 7631 CrossRef CAS PubMed.
- K. Mimura, F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Appl. Phys. Lett., 2012, 101, 012901 CrossRef.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, J. Am. Chem. Soc., 2014, 136, 3716 CrossRef CAS PubMed.
- Y. Nakagawa, H. Kageyama, R. Matsumoto, Y. Oaki and H. Imai, Nanoscale, 2015, 7, 18471 RSC.
- T. Hiraide, H. Kageyama, Y. Nakagawa, Y. Oaki and H. Imai, Chem. Commun., 2016, 52, 7545 RSC.
- Y. Zhang and F. M. Liu, RSC Adv., 2015, 5, 66934 RSC.
- M. Agthe, K. Høydalsvik, A. Mayence, P. Karvinen, M. Liebi, L. Bergström and K. Nygård, Langmuir, 2015, 31, 12537 CrossRef CAS PubMed.
- Q. Shi, K.-J. Si, D. Sikdar, L. W. Yap, M. Premaratne and W. Cheng, ACS Nano, 2016, 10, 967 CrossRef CAS PubMed.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, Langmuir, 2015, 31, 6197 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |