Magneto-acceleration of Ostwald ripening in hollow Fe3O4 nanospheres†
Wei
Ding‡
ab,
Lin
Hu‡
c,
Zhigao
Sheng
*cd,
Jianming
Dai
*a,
Xuebin
Zhu
a,
Xianwu
Tang
a,
Zhenzhen
Hui
a and
Yuping
Sun
acd
aKey Laboratory of Materials Physics, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, People's Republic of China. E-mail: jmdai@issp.ac.cn
bUniversity of Science and Technology of China, Hefei 230026, People's Republic of China
cHigh Magnetic Field Laboratory, Chinese Academy of Sciences, Hefei 230031, People's Republic of China. E-mail: zhigaosheng@hmfl.ac.cn
dCollaborative Innovation Centre of Advanced Microstructures, Nanjing University, Nanjing 210093, People's Republic of China
First published on 16th June 2016
Abstract
The magnetic field-induced acceleration of the Ostwald ripening process was demonstrated firstly in the formation of hollow Fe3O4 nanospheres. Similar to the maintaining time, the magnetic field can act as an independent parameter to control the crystallite size, hollow structure, and pore size of nanostructures in the Ostwald ripening process.
Ostwald ripening, as a description for the coarsening and secondary re-crystallization process, was firstly discovered by Friedrich Wilhelm Ostwald in 1900.1 It has been observed in crystal growth for more than one century and plays an important role in catalyst sintering.2 Recently, the design and fabrication of hollow and porous nanostructures have attracted much attention in materials research because of their enhanced properties in the fields of catalysis,3 sensing,4 energy storage,5,6 photoactivity,7 and drug delivery8 owing to their large specific surface area and hollow interior space. Ostwald ripening, as a century-old phenomenon, provides a newer self-template-based method for the preparation of hollow and porous nanostructures in addition to the templating synthesis methods. For example, Zeng et al. used Ostwald ripening as a template-free route to prepare anatase TiO2 hollow spheres in 2004.9 Their subsequent works have also demonstrated that Ostwald ripening can indeed be a general mechanism for the fabrication of hollow spheres of metal oxides and sulfides in solution.10–13 Nowadays, Ostwald ripening has been proven as an effective synthetic strategy for the formation of interior spaces in hollow spherical and nonspherical structures of CoFe2O4,14 α-MnO2,15 SnO2,6 γ-MnS,16 CaTiO3,17etc.
Ostwald ripening has become a sophisticated approach for making highly complex nanostructures, but its complex matter's relocation mechanism and artificial modulation have been rarely explored, though it is the basis for the controllable material architecture in this field. In recent years, people have made a number of related attempts. For example, it was discovered that this ripening process can be promoted specifically by fluoride-mediated surface dissolution in the synthesis process of TiO2 and SnO2 hollow microspheres.18 In 2011, Wang et al. found that the longer maintaining time and higher reaction temperature can apparently favor matter relocation in the ripening process in the experiment of preparation of SiO2 hollow spheres.13 As an important thermodynamic factor, the magnetic field can affect the physical and chemical processes of materials in a non-contact form and has been introduced in the synthesis process to control the nanostructures.19–22 However, up to now, nanostructure fabrication using Ostwald ripening in a magnetic field and the magneto-effect on the ripening process have not been explored. In this study, we take mesoporous Fe3O4 as an example to demonstrate the effect of magnetic fields on the Ostwald ripening process for the first time. The acceleration of Ostwald ripening caused by magnetic fields was found in the preparation of hollow mesoporous Fe3O4 nanospheres.
Fe3O4 nanospheres were prepared by a solvothermal method. The products are denoted as S10h-0T, S24h-0T, S48h-0T, S10h-0.5T, S10h-1T and S10h-3T according to the maintaining time and applied magnetic fields (see the ESI†).
The powder X-ray diffraction (XRD) patterns confirm that all samples are well crystallized and no other phase is detectable (Fig. S1 and S2†). The field emission scanning electron microscopy (FE-SEM) images, shown in Fig. 1, verify that all the samples have a spherical shape with a homogeneous diameter of ∼250 nm (Fig. S3†). The shell of the nanospheres consists of a loosely packed aggregate of Fe3O4 nanoparticles and the size of those nanoparticles increases gradually with the increase in maintaining time (Fig. 1a–c). This is a typical feature of Ostwald ripening. Upon application of magnetic fields, it is interesting to find that the Fe3O4 nanoparticle size is enlarged with the magnetic field intensity increasing though the same time (10 h) was maintained (Fig. 1a and d–f).
To determine the size dispersion in detail, the size distribution of the Fe3O4 nanoparticles was estimated by taking the average size of 150 nanoparticles and fitting the resultant histogram by a Gaussian function (dotted lines). The typical results are shown in Fig. 1g and h. It was found that the central size was shifted from 27.2 to 48.8 nm and 27.2 to 44.0 nm as the maintaining time was prolonged from 10 to 48 h and the magnetic field was increased from 0 to 1 T, respectively. These results clearly confirmed that the effect of the magnetic field on the particle size of the mesoporous Fe3O4 spheres is similar to that of the maintaining time. In particular, the morphology of S10h-0.5T is similar to that of S24h-0T, and the morphology of S10h-1T is similar to that of S48h-0T. When the magnetic field exceeds 1 T, the particle size expands slowly. For instance, the particle size increases by only 2.5% (from 44.0 to 45.1 nm) when the magnetic field was enhanced from 1 to 3 T, which implies that the Ostwald ripening effect tends to be saturated in this case when the magnetic field is beyond 1 T.
In the Ostwald ripening process, “solid–solution–solid” mass transport takes place (Scheme 1). The Fe3O4 crystallites located in the outermost surface are larger and would grow at the expense of smaller ones inside through the transport as shown in Scheme 1.10,23 Consequently, such gradual outward migration of crystals would result in continuous expansion of interiorly void space within the nanospheres. The evolutionary steps of interior spaces for Fe3O4 spheres in our case were studied by transmission electron microscopy (TEM) as shown in Fig. 2, S4 and S5.† The S10h-0T sample shows a solid sphere structure with straight channel like mesoporous from the center of the sphere (Fig. 2a). With an increase in maintaining time, S24h-0T displays a star-shaped void space at the center of the sphere, and the straight channel-like mesopores become larger compared to those of S10h-0T (Fig. 2b). With time increasing further, the void spaces at the center of the spheres in S48h-0T become larger (Fig. 2c). With application of magnetic fields, an obvious expansion of void space was also found under the same maintaining time. Different from the solid sphere structure of the S10h-0T sample, a small void space starts to form at the center of the sphere in S10h-0.5T (Fig. 2d) and it becomes larger with the increase in magnetic field intensity (Fig. 2e and f). Furthermore, we note that the hollow structure of S10h-0.5T is nearly the same as that of S24h-0T, while the hollow structure of S10h-1T is also similar to that of S48h-0T.
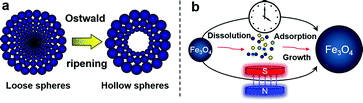 | ||
| Scheme 1 Schematic illustration of the Ostwald ripening process (a) and its magneto-acceleration effect (b). | ||
 | ||
| Fig. 2 TEM images of S10h-0T (a), S24h-0T (b), S48h-0T (c), S10h-0.5T (d), S10h-1T (e) and S10h-3T (f). | ||
In order to verify the magneto-acceleration effect further, the mesopore size distribution of Fe3O4 nanospheres were measured by Barrett–Joyner–Halenda (BJH) methods and the typical results are displayed in Fig. 3. The peak values of the pore size increase gradually from 4.6 to 6.7 and 9.2 nm when the maintaining time is prolonged from 10 to 24 and 48 h, respectively. Similarly, the peak values for the pore size distribution of S10h-0.5T and S10h-1T are found to be 7.1 and 9.1 nm, respectively. This magneto-enhancement of the pore size of Fe3O4 nanospheres, together with the XRD, FE-SEM and TEM results, suggests that the external magnetic field can significantly accelerate the Ostwald ripening process. When the external magnetic field exceeds the saturation value of 1 T, it is interesting to find that the pore size shrinks and is reduced down to 6.1 nm under the magnetic field of 3 T. This is probably because of the significant enhancement of mutually magnetic attraction between Fe3O4 particles in the higher magnetic field.
 | ||
| Fig. 3 Pore-size distribution curve of hollow Fe3O4 spheres obtained without (a) and with (b) magnetic fields. | ||
In the Ostwald ripening process, the Fe3O4 particles located in the outermost surface of aggregates grow at the expense of smaller ones inside, due to the higher solubility of the smaller particles (Gibbs–Thomson or Kelvin effect) and the molecular diffusion through the continuous phase (Scheme 1b).24 Gradual melting and outward migration of the inner particles would lead to continuous expansion of interior space (Scheme 1). With application of magnetic field, two aspects should be addressed. First, the applied magnetic field will produce a magnetic energy EM = −χVH2/2μ0, in which μ0 is the permeability of free space, χ is the volume magnetic susceptibility, V is the volume of Fe3O4 particles here, and H is the magnetic field intensity. The negative magnetic energy EM plays a crucial role in the surface energy reduction of the nanoparticles. Then, the larger Fe3O4 particles at the outermost surface, which possess large EM and lower surface energy, would be easier to produce in the ripening process.20 On the other hand, the participation of external magnetic fields will also affect the free energy (ΔG) of the ripening process directly. In addition to the original thermal Gibbs free energy ΔGT(T), the magnetic Gibbs free energy ΔGM(T, H) should be considered and the total Gibbs free energy of the ripening process becomes ΔG = ΔGT + ΔGM.25 ΔGM(T, H) can be written as −μ0(χa − χb)H2/2, in which χa and χb are the volume magnetic susceptibility of the magnetic particles after and before ripening, respectively. The smaller Fe3O4 nanoparticles have a diameter less than 30 nm in our case, which is very close to the size limitation of the superparamagnetic state (∼20 nm), and produce a negligible susceptibility χb.26 In the ripening process, the size enlargement of the Fe3O4 nanoparticles favors the stabilization of the ferromagnetic phase and a larger χa. The magnetization measurement of these Fe3O4 nanospheres indicates that the sample with larger particles has larger saturation magnetization (Fig. S6†). Consequently, a negative ΔGM is suggested which contributes to such magneto-acceleration of Ostwald ripening according to the theory that the state variation of the material advances toward the direction where the free energy is the lowest.27 To clarify the intrinsic mechanism of the magnetic field effect, theoretical calculation and further experiments on such an issue should be carried out in the future.
Conclusions
In summary, we report the magnetic field-induced acceleration of Ostwald ripening in Fe3O4 nanospheres for the first time. As an independent parameter, the external magnetic field is found to be an alternative tool to control the crystallite size, hollow structure, and pore diameter of the mesoporous Fe3O4 nanostructures. Reduction of the surface energy of the nanospheres and the negative magnetic free energy caused by the external magnetic field were discussed to explain such an acceleration effect. Our results suggest that the use of a magnetic field can be a novel approach to control the fabrication of hollow and porous nanostructures with the advantages of a simple yet very efficient and contact-free method, which might be very useful for future technological applications.Acknowledgements
We gratefully acknowledge financial support from the National Science Foundation of China (NSFC; Grant No. U1232210, 11574316, U1532155, 21301178), the National Key Basic Research Project of China (Grant No. 2014CB931704), and the One Thousand Youth Talents Program of China.Notes and references
- W. Ostwald, Z. Phys. Chem., 1900, 34, 495–503 Search PubMed.
- P. Wynblatt and N. A. Gjostein, Prog. Solid State Chem., 1975, 9, 21 CrossRef.
- R. T. Dong, H. L. Wang, Q. Zhang, X. T. Xu, F. Wang and B. Li, CrystEngComm, 2015, 17, 7406–7413 RSC.
- D. W. Wang, S. S. Du, X. Zhou, B. Wang, J. Ma, P. Sun, Y. F. Sun and G. Y. Lu, CrystEngComm, 2013, 15, 7438–7442 RSC.
- L. Y. Dang, H. F. Ma, J. Y. Xu, Y. Jin, J. J. Wang, Q. Y. Lu and F. Gao, CrystEngComm, 2016, 18, 544–549 RSC.
- X. W. Lou, Y. Wang, C. L. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- P. W. Li, X. L. Yan, Z. Q. He, J. L. Ji, J. Hu, G. Li, K. Lian and W. D. Zhang, CrystEngComm, 2016, 18, 1752–1759 RSC.
- R. C. Lv, S. L. Gai, Y. L. Dai, F. He, N. Niu and P. P. Yang, Inorg. Chem., 2014, 53, 998–1008 CrossRef CAS PubMed.
- H. G. Yang and H. C. Zeng, J. Phys. Chem. B, 2004, 108, 3492–3495 CrossRef CAS.
- J. Li and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 15839–15847 CrossRef CAS PubMed.
- Y. Chang, J. J. Teo and H. C. Zeng, Langmuir, 2005, 21, 1074–1079 CrossRef CAS PubMed.
- B. Liu and H. C. Zeng, Small, 2005, 1, 566–571 CrossRef CAS PubMed.
- D. P. Wang and H. C. Zeng, Chem. Mater., 2011, 23, 4886–4899 CrossRef CAS.
- H. Zhang, C. Zhai, J. Wu, X. Ma and D. Yang, Chem. Commun., 2008, 5648–5650 RSC.
- B. X. Li, G. X. Rong, Y. Xie, L. F. Huang and C. Q. Feng, Inorg. Chem., 2006, 45, 6404–6410 CrossRef CAS PubMed.
- Y. H. Zheng, Y. Cheng, Y. S. Wang, L. H. Zhou, F. Bao and C. Jia, J. Phys. Chem. B, 2006, 110, 8284–8288 CrossRef CAS PubMed.
- X. F. Yang, J. X. Fu, C. J. Jin, J. Chen, C. L. Liang, M. M. Wu and W. Z. Zhou, J. Am. Chem. Soc., 2010, 132, 14279–14287 CrossRef CAS PubMed.
- J. G. Yu, H. T. Guo, S. A. Davis and S. Mann, Adv. Funct. Mater., 2006, 16, 2035–2041 CrossRef CAS.
- Y. Ikezoe, N. Hirota, J. Nakagawa and K. Kitazawa, Nature, 1998, 393, 750 CrossRef.
- J. Wang, Q. W. Chen, C. Zeng and B. Y. Hou, Adv. Mater., 2004, 16, 137–140 CrossRef CAS.
- L. Hu, R. R. Zhang and Q. W. Chen, Nanoscale, 2014, 6, 14064 RSC.
- K. J. Zhang, J. M. Dai, X. B. Zhu, X. G. Zhu, X. Z. Zuo, P. Zhang, L. Hu, W. J. Lu, W. H. Song, Z. G. Sheng, W. B. Wu, Y. P. Sun and Y. W. Du, Sci. Rep., 2016, 6, 19483 CrossRef CAS PubMed.
- C. Y. Christopher and H. C. Zeng, J. Mater. Chem. A, 2014, 2, 4843–4851 RSC.
- Y. De Smet, L. Deriemaeker and R. Finsy, Langmuir, 1999, 15, 6745–6754 CrossRef CAS.
- J. H. Wang, Y. W. Ma and K. Watanabe, Chem. Mater., 2008, 20, 20–22 CrossRef CAS.
- C. J. Xu and S. H. Sun, Adv. Drug Delivery Rev., 2013, 65, 732–743 CrossRef CAS PubMed.
- K. Ichikawa and S. Shiratori, Inorg. Chem., 2011, 50, 999–1004 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures; XRD patterns, FE-SEM images, and magnetization of Fe3O4 nanospheres. See DOI: 10.1039/c6ce01021k |
| ‡ W. D. and L. H. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |

