Coordination of alkaline-earth metal cations to a symmetrical octamethyl-substituted cucurbituril in the presence of polychlorido cadmium(ii) anions†
Abstract
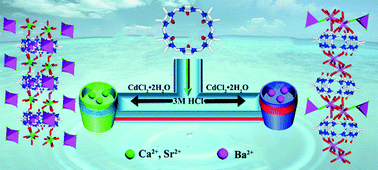
The interaction of the alkaline-earth metal ions Mg2+, Ca2+, Sr2+ and Ba2+ with the symmetrical octamethyl-substituted cucurbituril, OMeQ[6], in the presence of [CdCl4]2−/[Cd2Cl8]4− anions as structure-directing agent(s) in 3 M HCl has been investigated. Ca2+ and Sr2+ yielded closely related metallosupramolecular assemblies whose X-ray structures show that in the former, [Cd2Cl8]4− anions are arranged to form parallelogram-like frameworks that surround linear OMeQ[6]/Ca2+ polymeric units. The structure of the OMeQ[6]/Sr2+ assembly is similar except that the [Cd2Cl8]4− anions in the OMeQ[6]/Ca2+ assembly are replaced by [Cd2(H2O)2Cl8]4− anions. In contrast to the above structures, interaction of the larger Ba2+ ion with OMeQ[6] gives rise to a different assembly type displaying a porous linear polymeric arrangement incorporating two different Ba2+ coordination modes; [Cd2Cl4]4− anions are associated with the Ba2+ bridges linking adjacent OMeQ[6] units in the polymeric chain. In contrast to Ca2+ and Sr2+, the smaller Mg2+ ion under similar synthetic conditions failed to yield a solid product. Isothermal titration calorimetry (ITC) studies revealed that OMeQ[6] interacts with Ca2+, Sr2+ and Ba2+, but not Mg2+, in neutral aqueous solution. The respective X-ray structures reveal the presence of both channels and other voids in the three assemblies. Following application of a vacuum until constant weight was obtained, samples of the Sr2+ and Ba2+ assemblies were subjected to the saturated vapour of each of the following volatile ligands: acetonitrile, methanol, ethanol, acetone, diethylether, dichloromethane, cyclohexane and n-hexane. The respective absorption profiles show maximum absorption for methanol, with reduced uptake for the remaining larger and/or less polar guest molecules.
- This article is part of the themed collection: Form and Function of Molecular Cups and Capsules

 Please wait while we load your content...
Please wait while we load your content...