The structural effects of alkaline- and rare-earth element incorporation into thorium molybdates†
Bin
Xiao
ab,
Hartmut
Schlenz
a,
Dirk
Bosbach
a,
Evgeny V.
Suleimanov
c and
Evgeny V.
Alekseev
*ab
aInstitute for Energy and Climate Research (IEK-6), Forschungszentrum Jülich GmbH, 52428 Jülich, Germany
bInstitut für Kristallographie, RWTH Aachen University, 52066 Aachen, Germany. E-mail: e.alekseev@fz-juelich.de
cDepartment of Chemistry, Lobachevsky State University of Nizhny Novgorod, 603950, Nizhny Novgorod, Russia
First published on 12th November 2015
Abstract
Four novel thorium molybdates containing alkaline-earth or rare-earth metals were isolated from high-temperature solid-state synthesis. The incorporation of divalent and trivalent cations into the thorium molybdate system results in complex structural topologies. A2MgTh3(MoO4)8 (A = K, Rb) which crystallizes in the space group C2/c is the first instance among the thorium molybdate family that incorporates alkaline-earth metals. Its crystal structure is based on complex channels composed of ThO8 square antiprisms and MoO4 tetrahedra arranged in a corner-sharing manner. K2SrTh2(MoO4)6, as the first thorium polymolybdate compound, is constructed from ThO8 square antiprisms and Mo4O16 tetramers. The Mo4O16 tetramers, lying in the (001) plane, are built from four edge-sharing MoO6 octahedra. Nd2Th3(MoO4)9 is the first thorium molybdate containing rare-earth cations. The resemblance of Nd2Th3(MoO4)9 with hexagonal-ThMo2O8 reveals its potential as a host for different trivalent transuranium elements. Raman spectra analysis shows that the different Mo polyhedral geometries (MoO4 tetrahedra and MoO6 octahedra) have significant effects on the vibrational bands of these compounds. The thermal behavior and stability of the newly obtained materials have been studied.
Introduction
The chemical behaviour of synthetic and natural actinide molybdates has received considerable attention for a few decades due to their important roles in nuclear safety management.1 Molybdenum, in most cases, is one of the significant fission products produced in the spent nuclear fuel.2–4 The chemical reactions between actinide elements and molybdenum may produce complex compounds and these compounds could affect the nuclear fuel behaviour.5,6 In addition, actinide molybdates are considered as important materials for the long-term evolution of a geological repository for the spent nuclear fuel.7–12 From a more fundamental perspective, actinide molybdates bear complex structures and are capable of forming diverse topological configurations. In the past few decades, extended studies on actinide molybdate compounds have been mostly focused on high-valent uranium(VI) phases consisting of approximately linear uranyl cations UO22+.13–16 The uranyl UO22+ exhibits various coordination geometries, because of its ability to link four to six equatorial ligands and form tetragonal, pentagonal and hexagonal bipyramidal configurations. Based on this, a vast number of uranium molybdates incorporating alkali,14,17–19 alkaline-earth,4,20 transition21–24 and even lanthanide metals25 have already been isolated, which have exhibited exceedingly rich structural flexibility.In comparison, molybdate compounds containing lower-valent actinides such as Th(IV), Np(IV) and Pu(IV) are barely reported. These elements also exhibit a versatile structural chemistry. For example, the coordination number of Th(IV) can be varied from six to fifteen.26–28 Unlike most of the early actinides possessing various oxidation states, thorium is practically stable in tetravalent state Th(IV). This makes thorium much easier to handle, and thus it is frequently used as a surrogate for studying the chemical behaviour of more radiotoxic transuranic elements in valence state (IV) such as Pu(IV) and Np(IV).29
Most of the studies on thorium molybdates were carried out more than two decades ago.30–33 Recently, thorium has been considered in new generations of nuclear fuel cycles, which is spurring more attention on thorium compounds.34,35 The first reported thorium molybdate compound is orthorhombic ThMo2O8.36 It is based on corner-sharing ThO8 antiprisms and MoO4 tetrahedra. Using micro thermal analysis and powder X-ray diffraction accordingly, the initial work on quaternary thorium molybdate compounds containing alkali metals has been performed by Tabuteau and Pages.37 Thereafter, the crystal structures and some physicochemical properties of a series of thorium molybdate compounds, namely, K2Th(MoO4)3, K4Th(MoO4)4 as well as K8Th(MoO4)6 have been described by Huyghe et al.38–40 Recently, Dahale et al. presented the syntheses and thermal stabilities of Na2Th(MoO4)3 and Na4Th(MoO4)4.41 Subsequently, the study of thorium molybdates containing alkaline cations was completed by recently reported Rb–Th–Mo (ref. 42) and Cs–Th–Mo (ref. 43) families. In addition to these synthetic compounds, Orlandi et al. discovered the first two natural thorium molybdate hydrates, Th(MoO4)2·3H2O (ichnusaite)44 and Th(MoO4)2·H2O (nuragheite).45 The structures of these two minerals are based on ThO9 and MoO4 polyhedra, which are connected to each other by corner-sharing. However, besides the above mentioned pure thorium molybdates or alkaline thorium molybdates, no other phases are reported from this system.
In order to develop a better understanding of the structural chemistry of actinides with the purpose of modelling the complicated processes in nuclear waste disposal sites, we have undertaken a series of phase synthesis and characterization studies of thorium compounds containing hexavalent cations (S6+, Se6+, Te6+, Cr6+, W6+, Mo6+).13,43,46–54 We found that, compared to uranium molybdates, which favorably form 2D sheets,50,51 the thorium counterparts are structurally more versatile. This can be exemplified by one family of thorium molybdates, Rb–Th–Mo, where all compounds share common polyhedral units of MoO4 tetrahedra and ThO8 antiprisms but adopt different structural dimensionalities, from simple clusters in Rb8Th(MoO4)6, to a complex three-dimensional framework in Rb4Th5(MoO4)12. Continuing this study, here we perform an investigation of the thorium molybdate family that contains alkaline-earth metals and rare-earth metals. In this report, we discussed the influence of alkali- and rare earth-elements on the structures and stability of thorium molybdates.
Experimental section
Syntheses
The four titled thorium molybdate compounds were prepared by a solid-state reaction method using A.R. grade chemicals Th(NO3)4·5H2O (Merck) and MoO3 (Alfa-Aesar) without further purification. Th used in this study is an α-emitting radioisotope and thus standard precautions for handling radioactive materials should be strictly obeyed at all times.Single crystal preparation of K2MgTh3(MoO4)8
Th(NO3)4·5H2O (A.R. grade), KNO3 (A.R. grade), Mg(NO3)2 (A.R. grade) and MoO3 (A.R. grade) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were loaded into a Pt crucible after being fully ground. The mass weights of the reactants were 100.0 mg for Th(NO3)4·5H2O, 17.7 mg for KNO3, 52.0 mg for Mg(NO3)2 and 50.5 mg for MoO3. The Pt crucible was heated up to 1000 °C within 4 h in a furnace (CARBOLITE CWF 1300) and held constant at 1000 °C for 2 h in order to melt homogeneously. Afterwards, the mixture was cooled to 400 °C at a rate of 6.5 °C h−1, followed by quenching. The resulting products were washed with hot water and bulk colourless crystals of good quality were obtained. Due to the undistinguishable optical behaviour of the synthesized crystals and glassy pieces, the yield couldn't be calculated.
2 were loaded into a Pt crucible after being fully ground. The mass weights of the reactants were 100.0 mg for Th(NO3)4·5H2O, 17.7 mg for KNO3, 52.0 mg for Mg(NO3)2 and 50.5 mg for MoO3. The Pt crucible was heated up to 1000 °C within 4 h in a furnace (CARBOLITE CWF 1300) and held constant at 1000 °C for 2 h in order to melt homogeneously. Afterwards, the mixture was cooled to 400 °C at a rate of 6.5 °C h−1, followed by quenching. The resulting products were washed with hot water and bulk colourless crystals of good quality were obtained. Due to the undistinguishable optical behaviour of the synthesized crystals and glassy pieces, the yield couldn't be calculated.
Single crystal preparation of Rb2MgTh3(MoO4)8
Rb2MgTh3(MoO4)8 was prepared by heating a mixture of Th(NO3)4·5H2O (100.0 mg), RbNO3 (25.9 mg), Mg(NO3)2 (52.0 mg) and MoO3 (50.5 mg) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in a Pt boat. The remaining experimental conditions were identical to K2MgTh3(MoO4)8. After being washed with hot water, lots of colourless crystals with prismatic shape were isolated. The yield couldn't be obtained because of the undistinguishable shapes between the crystals and glassy pieces.
2 in a Pt boat. The remaining experimental conditions were identical to K2MgTh3(MoO4)8. After being washed with hot water, lots of colourless crystals with prismatic shape were isolated. The yield couldn't be obtained because of the undistinguishable shapes between the crystals and glassy pieces.
Single crystal preparation of K2SrTh2(MoO4)6
The reactants Th(NO3)4·5H2O (100.0 mg), KNO3 (53.2 mg), Sr(NO3)2 (148.5 mg) and MoO3 (50.5 mg) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were loaded into a Pt crucible. The mixture was ground and heated in a furnace at 950 °C for 3 h. The heating rate was 500 °C h−1 and the cooling rate was 5 °C h−1. The resultant mixture in the crucible consisted of well-formed colourless prisms of K2SrTh2(MoO4)6 as well as crystals of K2MoO4 and other undefined amorphous products.
2 were loaded into a Pt crucible. The mixture was ground and heated in a furnace at 950 °C for 3 h. The heating rate was 500 °C h−1 and the cooling rate was 5 °C h−1. The resultant mixture in the crucible consisted of well-formed colourless prisms of K2SrTh2(MoO4)6 as well as crystals of K2MoO4 and other undefined amorphous products.
Single crystal preparation of Nd2Th3(MoO4)9
Th(NO3)4·5H2O (100.0 mg), Nd2O3 (23.6 mg) and MoO3 (20.2 mg) with a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were loaded into a Pt crucible after being fully ground in an agate mortar. The crucible was then heated in a furnace for 6 h at 1100 °C, and slowly cooled to 400 °C at a rate of 5 °C h−1 followed by quenching. The resulting products consisted of pale violet Nd2Th3(MoO4)9 crystals and a glassy mass that was subsequently isolated. The yield couldn't be determined because of the similar shapes between the crystals and glass pieces.
4 were loaded into a Pt crucible after being fully ground in an agate mortar. The crucible was then heated in a furnace for 6 h at 1100 °C, and slowly cooled to 400 °C at a rate of 5 °C h−1 followed by quenching. The resulting products consisted of pale violet Nd2Th3(MoO4)9 crystals and a glassy mass that was subsequently isolated. The yield couldn't be determined because of the similar shapes between the crystals and glass pieces.
Preparation of the pure phases
All the obtained thorium molybdates were also prepared in the form of pure polycrystalline powder via standard solid-state methods. Using the same reagents and devices as for the single crystal syntheses, the pure thorium molybdate powders were prepared for each stoichiometric ratio. The procedure was as follows: after being thoroughly ground, the reaction mixtures were initially heated in air up to 400 °C and kept at this temperature for 30 h. After that, room-temperature X-ray powder diffraction analysis was required to analyze the phase content and purity. The same grinding and heating steps were repeated several times, increasing the temperature stepwise (50 °C per step), until pure phases were obtained. Finally, the pure phases of K2MgTh3(MoO4)8, Rb2MgTh3(MoO4)8, K2SrTh2(MoO4)6 and Nd2Th3(MoO4)9 could be prepared at temperatures starting at 550 °C, 600 °C, 650 °C and 600 °C, respectively.Crystallographic studies
The crystals of thorium molybdates selected for data collection were mounted on glass fibres and then optically aligned on an Agilent SuperNova (Dual Source) diffractometer. All data were collected using monochromatic Mo-Kα radiation with an incident wavelength of 0.71073 Å, running at 50 kV and 0.8 mA providing a beam size of approximately 30 μm in diameter. More than a hemisphere of data was collected for each crystal, and the three-dimensional data were reduced and outliers identified using the software CrysAlisPro. Data were corrected for Lorentz, polarization, absorption and background effects. The SHELXL-97 program was used for the determination and refinement of the structures.55 The data and crystallographic information are given in Table 1. The structures were solved by direct methods and refined to R1 = 0.020 for K2MgTh3(MoO4)8, R1 = 0.034 for Rb2MgTh3(MoO4)8, R1 = 0.053 for K2SrTh2(MoO4)6 and R1 = 0.042 for Nd2Th3(MoO4)9.| Compound | K2MgTh3(MoO4)8 | Rb2MgTh3(MoO4)8 | K2SrTh2(MoO4)6 | Nd2Th3(MoO4)9 |
|---|---|---|---|---|
| Mass | 2078.15 | 2170.89 | 1589.54 | 2424.03 |
| Space group | C2/c | C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P63/m |
| a (Å) | 18.568(7) | 18.6792(4) | 8.0197(10) | 17.556(3) |
| b (Å) | 18.0282(19) | 18.0812(5) | 8.4470(17) | 17.556(3) |
| c (Å) | 9.4188(14) | 9.4616(3) | 16.030(2) | 6.2713(10) |
| α (deg) | 90 | 90 | 101.550(15) | 90 |
| β (deg) | 90.35(3) | 90.230(2) | 102.893(12) | 90 |
| γ (deg) | 90 | 90 | 97.388(13) | 120 |
| V (Å3) | 3152.9(13) | 3195.56(15) | 1019.9(3) | 1673.9(7) |
| Z | 4 | 4 | 2 | 1 |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| F(000) | 3648.0 | 984.0 | 1400.0 | 1952.0 |
| μ (cm−1) | 17.546 | 20.078 | 21.194 | 19.716 |
| ρ calcd (g cm−3) | 4.378 | 4.512 | 5.176 | 4.820 |
| R(F) for Fo2 > 2σ(Fo2) | 0.0200 | 0.0342 | 0.0526 | 0.0414 |
| wR2 (Fo2) | 0.0434 | 0.0611 | 0.1300 | 0.1435 |
Powder X-ray diffraction
X-ray powder diffraction patterns were collected on a Bruker-AXS D4 Endeavor diffractometer in the Bragg–Brentano geometry, equipped with a copper tube and a primary nickel filter providing Cu K![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/char_e0c2.gif) radiation (λ = 1.54187 Å). A one-dimensional silicon strip LynxEye detector (Bruker-AXS) was used for data collection. A voltage of 40 kV and an electric current of 40 mA (1.6 kW) were set for the experiment. Data were recorded in the range of 2θ = 10–80° (total counting time = 10 s per step with the step width of 0.02°). The aperture of the fixed divergence slit was set to 0.2 mm and that of the receiving slit to 8.0 mm. The discriminator of the detector was set to an interval of 0.16 to 0.25 V. The X-ray powder diffraction patterns for K2MgTh3(MoO4)8, Rb2MgTh3(MoO4)8, K2SrTh2(MoO4)6 and Nd2Th3(MoO4)9 are provided in the ESI† (Fig. SI1).
radiation (λ = 1.54187 Å). A one-dimensional silicon strip LynxEye detector (Bruker-AXS) was used for data collection. A voltage of 40 kV and an electric current of 40 mA (1.6 kW) were set for the experiment. Data were recorded in the range of 2θ = 10–80° (total counting time = 10 s per step with the step width of 0.02°). The aperture of the fixed divergence slit was set to 0.2 mm and that of the receiving slit to 8.0 mm. The discriminator of the detector was set to an interval of 0.16 to 0.25 V. The X-ray powder diffraction patterns for K2MgTh3(MoO4)8, Rb2MgTh3(MoO4)8, K2SrTh2(MoO4)6 and Nd2Th3(MoO4)9 are provided in the ESI† (Fig. SI1).
Raman studies
Unpolarized Raman spectra were recorded with a Horiba LabRAM HR spectrometer using a Peltier cooled multi-channel CCD detector. An objective lens with a 50× magnification was linked to the spectrometer, allowing the analysis of samples as small as 2 μm in diameter. All the samples were in the form of polycrystalline powders. The incident radiation was produced by a He–Ne laser at a power of 17 mW (λ = 632.81 nm). The focal length of the spectrometer was 800 mm and an 1800 grooves per mm grating was used. The spectral resolution was around 1 cm−1 with a slit of 100 μm. The spectra were recorded in the range of 100–1050 cm−1. No photoluminescence (PL) was observed.Scanning electron microscopy/energy-dispersive spectroscopy (SEM/EDS)
Scanning electron microscopy images and energy-dispersive X-ray spectroscopy (SEM/EDS) data were collected on a FEI Quanta 200 F Environmental Scanning Electron Microscope. The instrument is equipped with an Apollo Silicon Drift Detector (SSD) from EDAX. The measurements were carried out in a low-vacuum mode at 60 Pa (30 kV, spot size 4, working distance 10 mm). The EDS results shown in the ESI† (Fig. S2 and Table S1) are in good agreement with the expected chemical compositions for all four thorium compounds.Thermal analysis
The thermal behaviour of the dried powders was studied from room temperature up to 1250 °C by differential scanning calorimetry analysis (DSC) coupled with thermogravimetry (TG) in air, at a heating rate of 10 °C min−1, using a Netzsch STA 449C Jupiter apparatus. 20 mg of each sample were loaded in a platinum crucible which was closed with a platinum cover. During the measurements a constant air flow of 20–30 ml min−1 was applied.Results and discussion
Structure descriptions
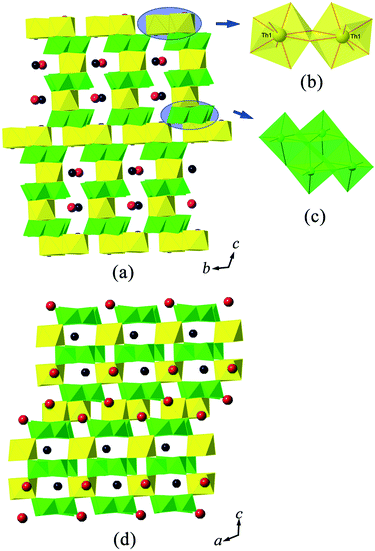
In the structure of A2MgTh3(MoO4)8 (A = K, Rb), all ThO8 square antiprisms are eight-fold coordinated by MoO4 tetrahedra. The local coordination environment of ThO8 in K2MgTh3(MoO4)8 that is chosen as an example is exhibited in Fig. 1(b). All O atoms of the Mo(1)O4 tetrahedra are bridging oxygen atoms to ThO8 polyhedra, whereas Mo(2)O4 and Mo(4)O4 each share three O atoms with ThO8 polyhedra, and Mo(3)O4 only shares two O atoms with adjacent ThO8 polyhedra. As one can easily imagine, these non-shared terminal O atoms in Mo(2)O4, Mo(3)O4 and Mo(4)O4 directly lead to an open-framework structure with channels running along the [001] direction. The resulting channels, having an elliptical cross-section, are filled with Mg2+ cations. As can be seen from Fig. 1(a), additional small voids within the framework that are created by ThO8 square antiprisms corner sharing with MoO4 polyhedra are accompanied by K+ cations.
The most notable feature of A2MgTh3(MoO4)8 (A = K, Rb) is the unusual complex channels, highlighted in Fig. 1(a). Fig. 2(a and b) show the local connections of ThO8 and MoO4 polyhedra inside the cylindroid channels. Using the black and white nodal representation,58 the idealized unfolding version of the topological graph for such a Th–Mo channel is shown in Fig. 2(c). It is assembled with four-connected black nodes (Th) and two-connected white nodes (Mo). It is obvious that its topology is composed of so-called basic building tapes (highlighted in Fig. 2(c)).59 The adjacent building tapes are joined by Mo(3) cations. In order to obtain the channels observed in the structure of A3MgTh3(MoO4)8, one needs to fold and glue the corresponding building tapes by the following procedure: as a first step, label the equivalent points on the sides of the tapes by the letters a, b, c, d, e, f and g. Then fold the tape by joining the associated opposite sides (a–a′, b–b′, c–c′, d–d′, e–e′, f–f′ and g–g′), as demonstrated in Fig. 2(c and d). It is notable that the method of basic building tapes not only allows us to recognize the internal Th–Mo polyhedral connections along the channels, but is also helpful to understand the symmetry inside the channels.60 The basic tapes are related by a two-fold screw axis perpendicular to the channel direction. Due to the inversion centre, as required by the space group C2/c, the cylindroid channels demonstrate an achiral cylindroid topology within this structure. Such a method was also adopted by Krivovichev et al. to present the linkage of U and Mo polyhedra inside the channels of a rare open-framework structure of (NH4)4[(UO2)5(MoO4)7](H2O)5.61 In contrast to A2MgTh3(MoO4)8, the equivalent points on the sides of the tapes for (NH4)4[(UO2)5(MoO4)7](H2O)5 are not oppositely orientated to each other, thus their folding procedure results in a chiral U–Mo channel topology.
Structure of K2SrTh2(MoO4)6
K2SrTh2(MoO4)6 crystallizes in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , consisting of ThO8 square antiprisms and Mo4O16 tetramers. The crystal structure of K2SrTh2(MoO4)6 is exhibited in Fig. 3. There are six crystallographically unique Mo sites and two distinct Th sites. Each Mo cation is coordinated by six O atoms forming a MoO6 octahedral configuration. Th(1) and Th(2), with a square antiprismatic coordination geometry, reveal varying Th–O bond distances ranging from 2.34(1) Å to 2.56(2) Å, with the mean value being 2.44 Å, which is slightly larger than that observed in Cs2Th(MoO4)3 (average Th–O = 2.41 Å),43 but agrees well with values reported for many molybdates containing eight-fold coordinated Th.38,39,62 In K2SrTh2(MoO4)6, two Th(1)O8 polyhedra, related by an inversion centre, share a common O(8)–O(8) edge to construct a Th(1)2O14 dimeric unit, whereas the Th(2)O8 polyhedra are found to be isolated from neighbouring ThO8 polyhedra.
, consisting of ThO8 square antiprisms and Mo4O16 tetramers. The crystal structure of K2SrTh2(MoO4)6 is exhibited in Fig. 3. There are six crystallographically unique Mo sites and two distinct Th sites. Each Mo cation is coordinated by six O atoms forming a MoO6 octahedral configuration. Th(1) and Th(2), with a square antiprismatic coordination geometry, reveal varying Th–O bond distances ranging from 2.34(1) Å to 2.56(2) Å, with the mean value being 2.44 Å, which is slightly larger than that observed in Cs2Th(MoO4)3 (average Th–O = 2.41 Å),43 but agrees well with values reported for many molybdates containing eight-fold coordinated Th.38,39,62 In K2SrTh2(MoO4)6, two Th(1)O8 polyhedra, related by an inversion centre, share a common O(8)–O(8) edge to construct a Th(1)2O14 dimeric unit, whereas the Th(2)O8 polyhedra are found to be isolated from neighbouring ThO8 polyhedra.
The most interesting structural feature of K2SrTh2(MoO4)6 is the Mo4O16 tetramers lying in the ab plane. They are built by four MoO6 octahedra connecting with each other in an edge-sharing manner. Each Mo4O16 tetramer contains two kinds of bridging oxygen atoms, that is, μ2-oxo and μ3-oxo groups. The Mo–O bond distances within the MoO6 octahedra vary from 1.72(2) Å to 2.44(2) Å. The average Mo–O bond distance is 1.96 Å, with some lengthening observed for Mo-μ3O (around 2.15 Å). The O–Mo–O bond angles for O atoms in cis positions vary from 76.4(2)° to 106.4(3)°, and are far from an ideal right angle. To our best knowledge, such a Mo4O16 tetramer has not been reported in any actinide family before. However, it can be typically found in polyoxomatellate compounds,63,64 where Mo can be aggregated in corner-, edge- and even face-sharing environments to form large and complex 3D frameworks. It also has been reported as a fundamental structural unit in some simple structures, such as Ag2Mo2O7.65
Each Mo4O16 tetrameter shares 14 oxygen atoms by attachment to three Th(1)2O14 dimers on the one side and two Th(2)O8 square antiprisms on the other side. As a result, two types of channels can be found in the crystal structure of K2SrTh2(MoO4)6. The first one, with interior dimensions of approximately 11.5 × 1.9 Å, is formed propagating in the [100] direction. These channels are located within the Th–O–Mo anionic network and are occupied by the charge-compensating K+ and Sr2+ cations. Additional small channels with a rectangular shape are occupied by Sr2+ cations and can be seen clearly if one views along the [010] direction. These two kinds of channels intersect with each other, resulting in an open-framework structure.
Structure of Nd2Th3(MoO4)9
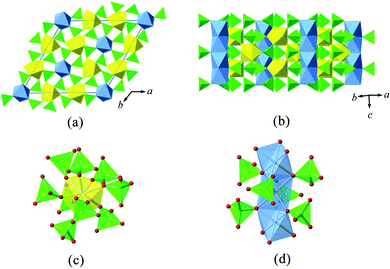
Nd2Th3(MoO4)9 crystallizes in the space group P63/m with a 3D framework structure, as shown in Fig. 4. The structure of Nd2Th3(MoO4)9 consists of one ThO9 site, two independent NdO6 sites, and three independent MoO4 sites. Each Th atom, coordinating to nine O atoms, connects to nine MoO4 tetrahedra forming a distorted and capped square antiprism coordination. The Th–O bond distances range from 2.42(1) to 2.51(1) Å, with an average bond distance equal to 2.44 Å.This bond distance is slightly shorter than those found in other synthetic thorium molybdates having nine-fold coordinated Th polyhedra, such as 2.46 Å in hexagonal ThMo2O8,66 but is still consistent with the ionic radii reported by Shannon.67 The Nd atoms are six-coordinated in a highly distorted octahedral geometry. The neighbouring Nd polyhedra are linked into the chains by sharing of two octahedral faces (Fig. 4(a) and (b)). As given in Fig. 4(d), each NdO6 octahedron is polarized by a three-fold rotation axis running through its parallel triangular faces. As a result, the Nd3+ cations move towards the octahedral face. This distortion is obviously reflected by a cis O–Nd–O configuration diverging from the ideal angle of 90°, exhibiting small (around 83°) and large (around 97°) angles. Each MoO4 tetrahedron is four-fold connected to both types of polyhedra, i.e. ThO9 and NdO6. The variation of Mo–O bond distances is appreciable, ranging from 1.70(1) to 1.81(1) Å, with the average distance ~1.75 Å. This is in good agreement with previous results of thorium molybdates in MoO4 tetrahedra.39,62
The structures of a series of compounds Ln2M3(MoO4)9 (Ln = La–Lu, Y, Sc; M = Zr, Hf)68,69 have a similar stoichiometric composition to Nd2Th3(MoO4)9. Both can be derived from the octahedral–tetrahedral framework of the mineral kosnarite (NaZr2(PO4)3), a structure type that has been proposed as a nuclear waste form due to its capacity of accommodating a large variety of radionuclides present in high-level wastes.70 Ln2M3(MoO4)9 is also based upon a 3D framework composed of three kinds of corner-sharing polyhedra: MoO4 tetrahedra, MO6 octahedra and nine-fold coordinated LnO9. One of the interesting features of Ln2M3(MoO4)9 compounds is that they have been demonstrated as an acceptor for immobilizing tri- and tetravalent actinides, which elucidates the chemical behaviour of actinides in processes of treatment and disposal of radioactive waste.71 As a new member of this family, Nd2Th3(MoO4)9 may also become a potential waste form, but further investigation involving Am3+ and Cm3+ is necessary in order to confirm this hypothesis.
It is also noteworthy that there is a close structural relation between Nd2Th3(MoO4)9 and hexagonal ThMo2O8. Both compounds show similar unit-cell parameters. The a and b parameters of Nd2Th3(MoO4)9 are shorter than those of hexagonal ThMo2O8, and this shortening can be explained by the inclusion of the smaller Nd3+ cation. Fig. 5 shows the polyhedral representations of both compounds. The similarity can best be demonstrated by projecting Nd2Th3(MoO4)9 and hexagonal-ThMo2O8 along each crystallographic c-axis. However, further structural analysis reveals that all six-coordinated sites (NdO6 octahedra) in Nd2Th3(MoO4)9 are connected via face-sharing, while the corresponding sites (ThO6 octahedra) observed in hexagonal ThMo2O8 are completely separated (Fig. 5(c) and (d), respectively). For this reason, the associated Nd–Nd bond distance in Nd2Th3(MoO4)9 is remarkably short, i.e. only around 3.1 Å. The fact that Nd2Th3(MoO4)9 and hexagonal-ThMo2O8 are based on a similar structural framework while containing different cations indicates that Th4+ cations can be partially substituted by trivalent ions while keeping the same structural skeleton. This means that such a framework has the ability to host diverse cations of various radii as well as valences without a considerable change of geometric parameters. Therefore, Nd2Th3(MoO4)9 can be used as a host phase for rare-earth elements and minor actinides (Am3+, Cm3+…).
Raman analysis
The Raman spectra of all thorium molybdates shown in Fig. 6 can be coarsely divided into two parts: a low frequency part between 100 cm−1 and 250 cm−1 in which the bands are associated with lattice phonon modes, and a high-frequency part from 250 cm−1 to 1000 cm−1, which is assigned to the internal motions of oxo-anion groups (MoO42− tetrahedron or MoO62− octahedron).72–76 As discussed in the structure analysis part, except for K2SrTh2(MoO4)6 which contains Mo4O16 tetramers constructed from edge-sharing of four MoO6 octahedra, all remaining units are based on separate MoO42− groups. For the MoO42− group, the vibrational data are well defined by a large number of available references.73,77–79 However, very little research has been undertaken so far on actinide molybdate materials. The free MoO42− tetrahedron has a Td symmetry and four normal modes which are assigned as A1(ν1), E(ν2), F1(rot), and F2(trans, ν3, ν4), where A1, E and F2 are Raman permitted whereas F2 is also IR active. ν1, ν2, ν3 and ν4 are denoted as non-degenerate symmetrical stretching mode, double degenerate symmetrical bending mode, and triple degenerate anti-symmetrical stretching and anti-symmetrical bending vibrations, respectively. The stretching vibrations ν1 and ν3 in ideal MoO42− are located in the region 700–1000 cm−1, while the bending vibrations (ν2 and ν4) are situated in the region 300–500 cm−1.76 When it comes to a specific structure, however, due to crystal field effects, the MoO42− groups are distorted, and therefore the number of possible modes is increased. Because of having a large number of atoms in an asymmetrical unit (e.g. 35 crystallographically different atoms observed in K2SrTh2(MoO4)6), the vibrational properties are remarkably complex for our reported compounds, thus here we only assign selected frequencies based on a comparison to literature data. The Raman-active shifts are listed in Table S2† and the complete Raman fitting results for each thorium molybdate can be found in the ESI† (Fig. S3).Like most of the reported molybdate compounds containing MoO4 tetrahedral units,77,80 strong vibrational bands for K2MgTh3(MoO4)8, Rb2MgTh3(MoO4)8 and Nd2Th3(MoO4)9 are observed related to the symmetric stretching modes (v1), while the asymmetric stretches (v3) are relatively weak. The Raman spectra of the isostructural compounds K2MgTh3(MoO4)8 and Rb2MgTh3(MoO4)8 are virtually identical. Both have a total of 276 normal vibrational modes (68Ag + 68Au + 70Bg + 70Bu) predicted by factor group analysis.81 Only minor vibrational differences occur between these two compounds. In particular, the highest peak located at 953(1) cm−1 in K2MgTh3(MoO4)8 can be assigned to the v1 of MoO4, while it is shifted to a lower frequency (951(1) cm−1) in Rb2MgTh3(MoO4)8. Such a distinction mainly originated from different Mo–O bond distances, which has been elucidated in detail by Hardcastle and Wachs.82 The most significant feature of Nd2Th3(MoO4)9 is the strong bands in the region from 750 cm−1 to 1000 cm−1. The high and sharp peak located at 965(1) cm−1 can be assigned to the symmetrical stretching mode v1 of the MoO42− tetrahedron. As for K2SrTh2(MoO4)6, because of the large number of atoms in an asymmetrical unit and Mo–O–Mo connections, the Raman profile shows complex broad and overlapping bands, originating from varying Mo–O bond distances and Mo–O–Mo bond angles.83 The stretching modes of K2SrTh2(MoO4)6 occur at wavenumbers from 700 to 1000 cm−1, and the bending modes from 250 to 450 cm−1, which is comparable to other molybdates containing MoO6 octahedra.83–85
Thermal analysis
Fig. 7 shows DSC and TG measurements of as-synthesized pure thorium molybdate phases. Black lines represent the DSC curves and blue lines the percentage mass loss (TG). The DSC curves exhibit different profiles for thorium molybdates containing different types of cations. The thermal behaviour of K2MgTh3(MoO4)8 is quite similar to that of alkaline thorium molybdates, such as Rb4Th5(MoO4)12 (ref. 42) and Na4Th(MoO4)4.41 K2MgTh3(MoO4)8 reveals two endothermic peaks, at around 921(5) °C and 1116(5) °C. The first peak indicates sample melting, and the broad and weak one, appearing immediately after the start of mass loss, shows the incongruent melting behaviour. A similar thermal evolution has also been observed for the isostructural counterpart Rb2MgTh3(MoO4)8. For K2SrTh2(MoO4)6 the DSC curve shows a strong endothermic peak at 985(5) °C indicating decomposition. For Nd2Th3(MoO4)9 decomposition seems to start at 667(5) °C, detectable by powder X-ray diffraction while heating the sample above this temperature. The main decomposition products can be indexed as hexagonal ThMo2O8 (see ESI,† Fig. S4). The associated mass loss due to MoO3 evaporation in this range can serve as additional proof.Conclusion
In conclusion, the isolation of a series of alkaline-earth or rare-earth thorium molybdates allows the elucidation of the influence of divalent and trivalent cations on the structural and physiochemical properties of thorium molybdates. Generally, the structural and thermal stability disparities between the thorium molybdates are strongly attributed to the radii and charge of the counter cations. In comparison with thorium molybdate (ThMo2O8), the thorium molybdate compounds with alkaline-earth or rare-earth metals are more complex. Specifically, A2MgTh3(MoO4)8 (A = K, Rb) are based on unusual complex cylindroid channels composed of ThO8 square antiprisms and MoO4 tetrahedra. Up to now, all known thorium molybdates are exclusively built by MoO4 tetrahedra. K2SrTh2(MoO4)6 is the first phase being constructed from MoO6 octahedral units. Nd2Th3(MoO4)9, comparable to octahedral–tetrahedral based natural kosnarite, has a structure similar to that of hexagonal ThMo2O8 but with NdO6 instead of ThO6 octahedra. The lanthanide to actinide substitution observed within the Nd2Th3(MoO4)9 framework may be an indication of the potential of this structure as a matrix for the immobilization of actinides.The DSC measurements demonstrate that all the studied compounds in this work exhibit high thermal stability. Unlike the simple thorium molybdate ThMo2O8, which exhibits tremendous polymorphic diversity with three different polymorphs stabilized at ambient pressure, no phase transition was observed for alkaline-earth or rare-earth thorium molybdates up to the associated melting temperature. Nd2Th3(MoO4)9 starts to decompose upon melting at around 667(5) °C; this is the lowest melting point observed for all known alkaline-earth or alkali metal thorium molybdates.
Associated content
X-ray crystallographic files for K2MgTh3(MoO4)8, K2SrTh2(MoO4)6, Nd2Th3(MoO4)9, and Rb2MgTh3(MoO4)8 in CIF format (CSD depository number 430398-430401).Acknowledgements
The authors are grateful to Dr. Martina Klinkenberg (IEK-6, Forschungszentrum Jülich) for her kind help in electron microscopy and EDX experiments. We are also grateful to the Helmholtz Association for funding within the VH-NG-815 grant.Notes and references
- P. C. Burns and R. J. Finch, Uranium: mineralogy, geochemistry and the environment, Mineralogical Society of America, 1999 Search PubMed.
- E. V. Alekseev, S. V. Krivovichev, T. Malcherek and W. Depmeier, Inorg. Chem., 2007, 46, 8442–8444 CrossRef CAS PubMed.
- S. V. Krivovichev, C. L. Cahill and P. C. Burns, Inorg. Chem., 2001, 41, 34–39 CrossRef.
- R. E. Marsh, J. Solid State Chem., 1988, 73, 577–578 CrossRef CAS.
- S. Dash, Z. Singh, N. D. Dahale, R. Prasad and V. Venugopal, J. Alloys Compd., 2002, 347, 301–307 CrossRef CAS.
- M. Keskar, K. Krishnan and N. D. Dahale, J. Alloys Compd., 2008, 458, 104–108 CrossRef CAS.
- C. C. Underwood, M. Mann, C. D. McMillen and J. W. Kolis, Inorg. Chem., 2011, 50, 11825–11831 CrossRef CAS PubMed.
- S. V. Krivovichev and P. C. Burns, J. Solid State Chem., 2002, 168, 245–258 CrossRef CAS.
- S. V. Krivovichev, R. J. Finch and P. C. Burns, Can. Mineral., 2002, 40, 193–200 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, T. Armbruster, W. Depmeier, E. V. Suleimanov, E. V. Chuprunov and A. V. Golubev, Z. Anorg. Allg. Chem., 2007, 633, 1979–1984 CrossRef CAS.
- J. N. Cross, P. M. Duncan, E. M. Villa, M. J. Polinski, J.-M. Babo, E. V. Alekseev, C. H. Booth and T. E. Albrecht-Schmitt, J. Am. Chem. Soc., 2013, 135, 2769–2775 CrossRef CAS PubMed.
- E. V. Suleimanov, A. V. Golubev, E. V. Alekseev, C. A. Geiger, W. Depmeier and V. G. Krivovichev, J. Chem. Thermodyn., 2010, 42, 873–878 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, T. Malcherek and W. Depmeier, Inorg. Chem., 2007, 46, 8442–8444 CrossRef CAS PubMed.
- E. V. Alekseev, E. V. Suleimanov, E. V. Chuprunov, A. V. Golubev, G. K. Fukin and M. O. Marychev, Russ. J. Inorg. Chem., 2007, 52, 1446–1449 CrossRef.
- S. V. Krivovichev, C. L. Cahill and P. C. Burns, Inorg. Chem., 2003, 42, 2459–2464 CrossRef CAS PubMed.
- S. Obbade, S. Yagoubi, C. Dion, M. Saadi and F. Abraham, J. Solid State Chem., 2003, 174, 19–31 CrossRef CAS.
- S. V. Krivovichev and P. C. Burns, Solid State Sci., 2003, 5, 481–485 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, T. Armbruster, W. Depmeier, E. V. Suleimanov, E. V. Chuprunov and A. V. Golubev, Z. Anorg. Allg. Chem., 2007, 633, 1979–1984 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, T. Malcherek and W. Depmeier, Inorg. Chem., 2007, 46, 8442–8444 CrossRef CAS PubMed.
- M. R. Lee and S. Jaulmes, J. Solid State Chem., 1987, 67, 364–368 CrossRef CAS.
- D. Y. Pushcharovsky, R. K. Rastsvetaeva and H. Sarp, J. Alloys Compd., 1996, 239, 23–26 CrossRef CAS.
- S. V. Krivovichev and P. C. Burns, Inorg. Chem., 2002, 41, 4108–4110 CrossRef CAS PubMed.
- S. V. Krivovichev and P. C. Burns, Can. Mineral., 2003, 41, 1455–1462 CrossRef CAS.
- S. V. Krivovichev and P. C. Burns, Can. Mineral., 2003, 41, 707–719 CrossRef CAS.
- I. V. Tat'yanina, E. B. Fomicheva, V. N. Molchanov, V. E. Zavodnik, V. K. Bel'skii and E. A. Torchenkova, Kristallografiya, 1982, 27 Search PubMed.
- S. R. Daly, P. M. B. Piccoli, A. J. Schultz, T. K. Todorova, L. Gagliardi and G. S. Girolami, Angew. Chem., Int. Ed., 2010, 49, 3379–3381 CrossRef CAS PubMed.
- N. Yu, V. V. Klepov, P. Kegler, D. Bosbach, T. E. Albrecht-Schmitt and E. V. Alekseev, Inorg. Chem., 2014, 53, 8194–8196 CrossRef CAS PubMed.
- N. Yu, V. V. Klepov, G. Modolo, D. Bosbach, E. V. Suleimanov, T. M. Gesing, L. Robben and E. V. Alekseev, Inorg. Chem., 2014, 53, 11231–11241 CrossRef CAS PubMed.
- G. Wallez, P. E. Raison, N. Dacheux, N. Clavier, D. Bykov, L. Delevoye, K. Popa, D. Bregiroux, A. N. Fitch and R. J. M. Konings, Inorg. Chem., 2012, 51, 4312–4322 CrossRef PubMed.
- J. Thoret, Rev. Chim. Miner., 1974, 11, 237 CAS.
- N. E. A. Bushuev, Russ. J. Inorg. Chem., 1975, 20, 337 Search PubMed.
- N. Bushuev, Russ. J. Inorg. Chem., 1975, 20, 639 Search PubMed.
- N. Bushuev, Russ. J. Inorg. Chem., 1975, 20, 645 Search PubMed.
- R. Hargraves and R. Moir, Am. Sci., 2010, 98, 304 CrossRef.
- M. Kazimi, Am. Sci., 2003, 91, 408–415 CrossRef.
- W. Freundlich and M. Pagès, C. R. Acad. Sci., 1969, 269, 392–394 CAS.
- A. Tabuteau and M. Pages, J. Inorg. Nucl. Chem., 1980, 42, 401–403 CrossRef CAS.
- M. Huyghe, M. R. Lee, S. Jaulmes and M. Quarton, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 950–954 CrossRef.
- M. Huyghe, M. R. Lee, M. Quarton and F. Robert, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 244–246 CrossRef.
- M. Huyghe, M. R. Lee, M. Quarton and F. Robert, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 1797–1799 CrossRef.
- N. D. Dahale, M. Keskar and K. D. Singh Mudher, J. Alloys Compd., 2006, 415, 244–250 CrossRef CAS.
- B. Xiao, T. M. Gesing, P. Kegler, G. Modolo, D. Bosbach, H. Schlenz, E. V. Suleimanov and E. V. Alekseev, Inorg. Chem., 2014, 53, 3088–3098 CrossRef CAS PubMed.
- B. Xiao, J. Dellen, H. Schlenz, D. Bosbach, E. V. Suleimanov and E. V. Alekseev, Cryst. Growth Des., 2014, 14, 2677–2684 CAS.
- P. Orlandi, C. Biagioni, L. Bindi and F. Nestola, Am. Mineral., 2014, 99, 2089–2094 CrossRef.
- P. Orlandi, C. Biagioni, L. Bindi and S. Merlino, Am. Mineral., 2015, 100, 267–273 CrossRef.
- E. V. Alekseev, S. V. Krivovichev, T. Armbruster, W. Depmeier, E. V. Suleimanov, E. V. Chuprunov and A. V. Golubev, Z. Anorg. Allg. Chem., 2007, 633, 1979–1984 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, W. Depmeier, T. Armbruster, H. Katzke, E. V. Suleimanov and E. V. Chuprunov, J. Solid State Chem., 2006, 179, 2977–2987 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, W. Depmeier, T. Malcherek, E. V. Suleimanovl and E. V. Chuprunov, Z. Kristallogr., 2007, 222, 391–395 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev, W. Depmeier, O. I. Siidra, K. Knorr, E. V. Suleimanov and E. V. Chuprunov, Angew. Chem., Int. Ed., 2006, 45, 7233–7235 CrossRef CAS PubMed.
- E. V. Alekseev, E. V. Suleimanov, E. V. Chuprunov, A. V. Golubev, G. K. Fukin and M. O. Marychev, Russ. J. Inorg. Chem., 2007, 52, 1446–1449 CrossRef.
- E. V. Alekseev, E. V. Suleimanov, M. O. Marychev, E. V. Chuprunov and G. K. Fukin, J. Struct. Chem., 2006, 47, 881–886 CrossRef CAS.
- G. Wallez, P. E. Raison, N. Dacheux, N. Clavier, D. Bykov, L. Delevoye, K. Popa, D. Bregiroux, A. N. Fitch and R. J. M. Konings, Inorg. Chem., 2014, 53, 3088–3098 CrossRef PubMed.
- B. Xiao, T. M. Gesing, L. Robben, D. Bosbach and E. V. Alekseev, Chem. – Eur. J., 2015, 21, 7629–7629 CrossRef CAS PubMed.
- B. Xiao, T. M. Gesing, L. Robben, D. Bosbach and E. V. Alekseev, Chem. – Eur. J., 2015, 21, 7746–7754 CrossRef CAS PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- S. Wang, P. Yu, B. A. Purse, M. J. Orta, J. Diwu, W. H. Casey, B. L. Phillips, E. V. Alekseev, W. Depmeier, D. T. Hobbs and T. E. Albrecht-Schmitt, Adv. Funct. Mater., 2012, 22, 2241–2250 CrossRef CAS.
- P. Yu, S. Wang, E. V. Alekseev, W. Depmeier, D. T. Hobbs, T. E. Albrecht-Schmitt, B. L. Phillips and W. H. Casey, Angew. Chem., Int. Ed., 2010, 49, 5975–5977 CrossRef CAS PubMed.
- S. Krivovichev, P. Burns and I. Tananaev, Structural chemistry of inorganic actinide compounds, Elsevier, 2006 Search PubMed.
- S. Wang, E. V. Alekseev, J. Ling, G. Liu, W. Depmeier and T. E. Albrecht-Schmitt, Chem. Mater., 2010, 22, 2155–2163 CrossRef CAS.
- S. Wang, T. G. Parker, D. J. Grant, J. Diwu, E. V. Alekseev, W. Depmeier, L. Gagliardi and T. E. Albrecht-Schmitt, Inorg. Chem., 2012, 51, 11211–11213 CrossRef CAS PubMed.
- S. V. Krivovichev, C. L. Cahill and P. C. Burns, Inorg. Chem., 2003, 42, 2459–2464 CrossRef CAS PubMed.
- T. L. Cremers, P. G. Eller and R. A. Penneman, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 1165–1167 CrossRef.
- J. Zhao, D. Shi, L. Chen, P. Ma, J. Wang and J. Niu, CrystEngComm, 2011, 13, 3462–3469 RSC.
- A. J. Bridgeman and G. Cavigliasso, Polyhedron, 2002, 21, 2201–2206 CrossRef CAS.
- B. M. Gatehouse and P. Leverett, J. Chem. Soc., Dalton Trans., 1976, 1316–1320 RSC.
- E. M. Larson, P. G. Eller, T. L. Cremers, R. A. Penneman and C. C. Herrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1989, 45, 1669–1672 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- B. G. Bazarov, V. G. Grossman, R. F. Klevtsova, A. G. Anshits, T. A. Vereshchagina, L. A. Glinskaya, Y. L. Tushinova, K. N. Fedorov and Z. G. Bazarova, J. Struct. Chem., 2009, 50, 566–569 CrossRef CAS.
- R. F. Klevtsova, S. F. Solodovnikov, Y. L. Tushinova, B. G. Bazarov, L. A. Glinskaya and Z. G. Bazarova, J. Struct. Chem., 2000, 41, 280–284 CrossRef CAS.
- R. C. Ewing and L. Wang, Rev. Mineral. Geochem., 2002, 48, 673–699 CrossRef CAS.
- T. A. Vereshchagina, E. V. Fomenko, N. G. Vasilieva, L. A. Solovyov, S. N. Vereshchagin, Z. G. Bazarova and A. G. Anshits, J. Mater. Chem., 2011, 21, 12001–12007 RSC.
- J. Hanuza, M. M
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) czka, J. Lorenc, A. A. Kaminskii, L. Bohaty and P. Becker, J. Raman Spectrosc., 2010, 41, 424–430 CrossRef CAS.
czka, J. Lorenc, A. A. Kaminskii, L. Bohaty and P. Becker, J. Raman Spectrosc., 2010, 41, 424–430 CrossRef CAS. - J. Hanuza, M. M
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) czka, J. Lorenc, A. A. Kaminskii, L. Bohaty and P. Becker, J. Raman Spectrosc., 2010, 41, 424–430 CrossRef CAS.
czka, J. Lorenc, A. A. Kaminskii, L. Bohaty and P. Becker, J. Raman Spectrosc., 2010, 41, 424–430 CrossRef CAS. - K. Hermanowicz, M. Mączka, P. J. Dereń, J. Hanuza, W. Stręk and H. Drulis, J. Lumin., 2000, 92, 151–159 CrossRef CAS.
- M. M
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) aczka, W. Paraguassu, A. G. S. Filho, P. T. C. Freire, F. E. A. Melo, J. M. Filho and J. Hanuza, J. Raman Spectrosc., 2005, 36, 56–62 CrossRef.
aczka, W. Paraguassu, A. G. S. Filho, P. T. C. Freire, F. E. A. Melo, J. M. Filho and J. Hanuza, J. Raman Spectrosc., 2005, 36, 56–62 CrossRef. - V. P. Mahadevan Pillai, T. Pradeep, M. J. Bushiri, R. S. Jayasree and V. U. Nayar, Spectrochim. Acta, Part A, 1997, 53, 867–876 CrossRef.
- S. A. Klimin, M. N. Popova, B. N. Mavrin, P. H. M. van Loosdrecht, L. E. Svistov, A. I. Smirnov, L. A. Prozorova, H. A. K. von Nidda, Z. Seidov, A. Loidl, A. Y. Shapiro and L. N. Demianets, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 174408–174408 CrossRef.
- C. Cascales, M. D. Serrano, F. Esteban-Betegón, C. Zaldo, R. Peters, K. Petermann, G. Huber, L. Ackermann, D. Rytz, C. Dupré, M. Rico, J. Liu, U. Griebner and V. Petrov, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 174114–174114 CrossRef.
- F. J. Manjon, D. Errandonea, N. Garro, J. Pellicer-Porres, J. López-Solano, P. Rodríguez-Hernández, S. Radescu, A. Mujica and A. Muñoz, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 144112–144112 CrossRef.
- M. Maczka, W. Paraguassu, P. T. C. Freire, A. Majchrowski and P. S. Pizani, J. Phys.: Condens. Matter, 2013, 25, 125404–125404 CrossRef CAS PubMed.
- D. L. Rousseau, R. P. Bauman and S. P. S. Porto, J. Raman Spectrosc., 1981, 10, 253–290 CrossRef CAS.
- F. D. Hardcastle and I. E. Wachs, J. Raman Spectrosc., 1990, 21, 683–691 CrossRef CAS.
- V. O. Sokolov, V. G. Plotnichenko, V. V. Koltashev and I. A. Grishin, J. Non-Cryst. Solids, 2009, 355, 239–251 CrossRef CAS.
- G. A. Nazri and C. Julien, Solid State Ionics, 1992, 53–56(Part 1), 376–382 CrossRef CAS.
- L. Seguin, M. Figlarz, R. Cavagnat and J. C. Lassègues, Spectrochim. Acta, Part A, 1995, 51, 1323–1344 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, BSE image and EDS measuring points and Raman fitting results. CCDC 1430749–1430752. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ce02040a |
| This journal is © The Royal Society of Chemistry 2016 |