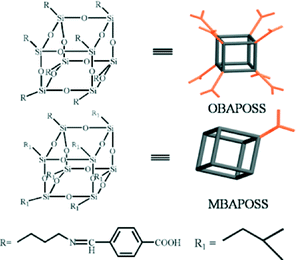
Synthesis and luminescence of octacarboxy cubic polyhedral oligosilsesquioxanes coordinated with terbium†
Qianqian
Xu
,
Zhiqiang
Li
,
Meng
Chen
and
Huanrong
Li
*
School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, PR China. E-mail: lihuanrong@hebut.edu.cn
First published on 9th November 2015
Abstract
Through the combination of polyhedral oligosilsesquioxane (POSS) and a lanthanide complex, a hybrid material with enhanced luminescent properties was achieved. FTIR, XRD, SEM, and photoluminescence spectroscopy were employed to characterize the obtained material. Improved photo- and thermal-stability were observed in the hybrid material when compared to the mono-modified POSS derivative and individual lanthanide complex. Modification at each silicon vertex of the POSS core effectively increased the lanthanide concentration that was doped in the hybrid material and the 3D divergent configuration of octa-benzoic acid functionalized POSS (OBAPOSS) ensured the uniform distribution of lanthanide in the hybrid material to prevent aggregation of the lanthanide complex at a high concentration. The combination of POSS with lanthanide used in this study for designing and preparing luminescent hybrid materials is simple and applicable, and has provided new facilities for the design of optical devices and the substitution of pure lanthanide complexes in crystal engineering. The unique luminescence properties of the hybrid material, together with its good stability and processability, make it an ideal candidate for a wide range of applications such as materials for flat-panel displays, luminescent probes in bioassays, UV sensors and as laser materials and optoelectronic devices.
Introduction
Trivalent lanthanide ions have attracted considerable attention over the past few decades due to their unique luminescence properties such as large Stokes shifts, sharp emission profiles, and long-lived excited states.1–10 The emission intensities of bare lanthanide ions are weak and are caused by the forbidden nature of the f-f transitions.11 Fortunately, this problem can be overcome by the so-called antenna effect (or sensitization)12–14 using certain organic ligands. To date, functional organic ligands, such as terpyridine,15 β-diketone,16 pyridine-2,6-dicarboxylate17 and phosphine oxides, have emerged as effective antenna molecules. Among them, β-diketone is most researched due to its good luminescence behavior. However, these compounds are usually too unstable for long-term use in devices. Even after being encapsulated into a matrix, their stability remains questionable.5 Therefore, it is strongly recommended to develop new ligand based luminescent lanthanide complexes rather than β-diketonate compounds. Aromatic carboxylic acids are regarded as an ideal candidate; however, a great deal of study needs to be carried out on designing carboxylates18 that can efficiently sensitize Ln3+.Polyhedral oligomeric silsesquioxane (POSS), with a Si8O12 cubic silica core and eight variable organic side groups appended on each silicon vertex of the cage,19–22 has been widely used as convenient and versatile building blocks in the construction of porous materials,23–25 catalysis,26–28 self-assembled nanoarchitectures29,30 and hybrid nanocomposite materials.31,32 POSS is considered as the smallest nanohybrid molecule, its rigid inorganic core provides good mechanical properties and high thermal/chemical stability. Moreover, the peripheral organic functional groups give rise to excellent solubility, compatibility with other materials and are easy to modify.33
Despite the extra ordinary properties provided by POSS, the use of lanthanide-coordinated organosilane precursors towards organic–inorganic hybrid materials has not been extensively explored. Recently, a europium(III) β-diketonate complex functionalized POSS by immobilization of the β-diketonate group at a silicon vertex of the POSS have been reported.34,35 The thermal stability and luminescence performance of (POSS-TTA)3Eu were also investigated; the luminescence efficiency is limited by the low lanthanide concentration doped in the hybrid material. Considering there are eight vertexes at the periphery of the POSS cores, modification at each silicon vertex with functional organic ligand would be an effective way to overcome this drawback. Herein, we report a luminescent organic–inorganic hybrid material obtained by complexing Tb3+ to octacarboxy functionalized POSS, which was constructed via grafting 8 benzoic acid (BA) moieties to each vertex of POSS. In addition, significant improvements in the luminescence performance, thermal- and photo-stability of the obtained hybrid materials were achieved. The octa-benzoic acid functionalized POSS (denoted as OBAPOSS) (Scheme 1) was prepared by reaction of 4-carboxybenzaldehyde with 8NH2-POSS via the Schiff base reaction (Scheme S1†). Mono-benzoic acid-functionalized POSS (denoted as MBAPOSS) was also synthesized as a reference compound using the same method (Scheme S2†).
Results and discussion
To evaluate the content of BA and silicon in OBAPOSS, we conducted TGA of OBAPOSS in an air atmosphere at a heating rate of 10 °C min−1 (Fig. S1†). The thermograms of OBAPOSS revealed a total mass loss of 74.3%, implying the formation of stable SiO2 (25.7%); this corresponded to a residue silicon yield of 11.99%, a value in good agreement with the theoretical value (calcd. 11.57%). This result indicates that eight vertexes of the POSS cores have been successfully modified with BA in OBAPOSS. The weight loss of MBAPOSS was 53% in the same temperature region, which revealed the content of BA and silicon in MBAPOSS were also closer to the theoretical values.The coordination behaviors of OBAPOSS and MBAPOSS with Tb3+ were investigated by Fourier transform infrared (FT-IR) spectroscopy. As shown in Fig. 1, the FT-IR spectra of OBAPOSS and Tb@OBAPOSS show peaks at 1110 cm−1, which can be attributed to the asymmetric stretching of Si–O–Si, and the band at 802 cm−1 can be assigned to Si–C rocking. In addition, two intense peaks at about 1610 cm−1 and 1630 cm−1 are observed in the OBAPOSS and MBAPOSS spectra, which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration (Fig. S2†). After coordination with Tb3+, the characteristic stretching vibrations of carboxyl groups at 1696 cm−1 almost completely disappeared. This phenomenon indicates that the Tb3+ was successfully coordinated to OBAPOSS. In addition, the bands at 1684 cm−1 belonging to the stretching vibrations of the carboxyl groups in MBAPOSS also disappeared, which implies the coordination between Tb3+ and MBAPOSS.
N stretching vibration (Fig. S2†). After coordination with Tb3+, the characteristic stretching vibrations of carboxyl groups at 1696 cm−1 almost completely disappeared. This phenomenon indicates that the Tb3+ was successfully coordinated to OBAPOSS. In addition, the bands at 1684 cm−1 belonging to the stretching vibrations of the carboxyl groups in MBAPOSS also disappeared, which implies the coordination between Tb3+ and MBAPOSS.
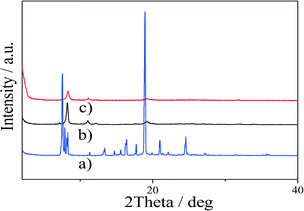
The formation of the lanthanide doped POSS cage was further confirmed by powder X-ray diffraction analysis (Fig. 2 and 3). The XRD revealed a somewhat broad pattern assigned to the amorphous structure arising from the loose molecular packing of OBAPOSS, which was caused by the substitution of isobutene with the aminopropyl and BA moieties. After coordination with Tb3+, a sharp pattern at 2θ = 8.3° with a d-spacing of 12.17 Å was observed.36 This spacing corresponds to the spacing of two adjacent POSS units bridged by the Tb(BA)3 (ref. 18) lanthanide complex in the unceasingly extended three-dimensional structure. On the other hand, MBAPOSS can only form a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 independent complex with Tb3+, and there was nearly no change observed in the XRD diffraction of Tb@OBAPOSS when compared to OBAPOSS. This deduction was confirmed by SEM with numerous nanospheres with diameters from 0.5 to 1 μm being observed in the SEM images of Tb@OBAPOSS. However, only amorphous morphological structures were observed in the presence of Tb@MBAPOSS (Fig. 4).
1 independent complex with Tb3+, and there was nearly no change observed in the XRD diffraction of Tb@OBAPOSS when compared to OBAPOSS. This deduction was confirmed by SEM with numerous nanospheres with diameters from 0.5 to 1 μm being observed in the SEM images of Tb@OBAPOSS. However, only amorphous morphological structures were observed in the presence of Tb@MBAPOSS (Fig. 4).
 | ||
| Fig. 4 Scanning electron micrographs for a) MBAPOSS, b) OBAPOSS, c) Tb@MBAPOSS and d) Tb@OBAPOSS (the scale bars are 4 μm, 5 μm, 1 μm and 2 μm, respectively). | ||
The luminescence properties of Tb@MBAPOSS, Tb@OBAPOSS and the pure complex Tb(BA)3 powder were characterized by their excitation and emission spectra at room temperature. A broad band ranging from 250 to 400 nm was observed in the excitation spectra of each sample and assigned to BA absorption (Fig. 5), indicating the occurrence of energy transfer from BA to Tb3+ and confirming the formation of a lanthanide complex between MBAPOSS/OBAPOSS and Tb3+ ions. The absorptions of Tb3+ were not observed, which indicated that the energy transfer from the ligand to Tb3+ was more efficient. The emission spectrum excited at 300 nm exhibit four sharp bands at 488, 544, 583, and 620 nm, ascribed to the 5D4 → 7FJ (J = 6–3) transitions of Tb3+. It was dominated by the 5D4 → 7F5 band at 544 nm and was responsible for the green emission color shown in Fig. 6. The emission intensity of Tb@OBAPOSS was stronger than that found for Tb@MBAPOSS and the pure complex. In addition, the quantum yield determined by the integrating sphere also increased about 2-fold, from 0.20 to 0.40. We also monitored the luminescence decays of our system, which show that the excited state of OBAPOSS was much longer than that found for Tb@MBAPOSS and Tb(BA)3; the luminescence decay was well represented by a mono-exponential (Fig. S3†). The average decay time for Tb(BA)3 with the 5D0 state was 0.51 ms and a significant change was observed for Tb@MBAPOSS (0.54 ms). However, the average decay time for Tb@OBAPOSS was significantly prolonged to 0.91 ms.
 | ||
| Fig. 5 Excitation spectra monitored at 544 nm and emission spectra excited at 300 nm of Tb(BA)3, Tb@MBAPOSS and Tb@OBAPOSS. | ||
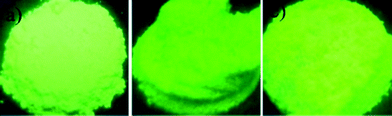 | ||
| Fig. 6 Digital images of a) Tb(BA)3, b) Tb@MBAPOSS and c) Tb@OBAPOSS under UV light illumination at 302 nm. | ||
As is well known, the thermal- and photo-stability of lanthanide complexes are extremely important criteria in the practical applications of photonic and optoelectronic devices. We therefore investigated the thermal- and photo-stability of the obtained luminescent hybrid materials. Changes in the emission intensity at the 5D4 → 7F5 line under UV light irradiation as a photo-stability study is illustrated in Fig. 7a. The results reveal that no significant loss of emission intensity at 5D4 → 7F5 was observed for Tb@OBAPOSS and Tb@MBAPOSS (Fig. S4b† and Fig. 7a inset) upon irradiation as the exposure time was increased, whereas the emission intensity of the corresponding individual complex Tb(BA)3 (Fig. S4a† and Fig. 7a inset) dramatically decreased to 40% of its original value after 20 h of UV-irradiation. This implies that both Tb@OBAPOSS and Tb@MBAPOSS exhibit good photo-stability and Tb@OBAPOSS was more stable than Tb@MBAPOSS. This instability of terbium(III) complexes towards UV-irradiation has already been reported,37–39 and the photo-stability improvement in our system was possibly due to the protection effect of the POSS scaffold. In addition, the 5D4 excited-state lifetime and the quantum yield of Tb3+ in Tb@MBAPOSS and Tb@OBAPOSS exhibited negligible change after 20 h of UV-irradiation, whereas the decay time for Tb(BA)3 decreased sharply (Tables S1 & S2†). The good luminescence properties of the hybrid material, such as long lifetime and high color purity, together with its good photo-stability and process ability, make it highly promising for use in flexible electronic applications.
 | ||
| Fig. 7 Changes in the emission luminescence spectra of Tb@OBAPOSS monitored at 544 nm at different exposure times: a) under UV light irradiation and b) at 120 °C. | ||
The thermo-stability of the luminescent hybrid materials was investigated by measuring the emission intensity after heating at 120 °C for 20 h. From Fig. 7b, we can observe that the luminescence emission of Tb@OBAPOSS lost 25% of its original intensity value after being heated at 120 °C for 10 h (Fig. 7b and 7b inset), whereas the value was 50% for Tb@MBAPOSS and 70% for Tb(BA)3 (Fig. S5† and Fig. 7b inset). These results demonstrate that Tb@OBAPOSS possesses better thermo-stability than Tb@MBAPOSS and the individual rare earth complex. The higher thermal stability of Tb@OBAPOSS over Tb@MBAPOSS may be attributed to the formation of the stable 3D network in Tb@OBAPOSS. To evaluate the thermal- and photo-stability of the hybrid materials, we have measured the XRD of the heated and illuminated samples, as shown in Fig. S6.† The XRD pattern of Tb@OBAPOSS remained almost the same, whereas the intensity of Tb@MBAPOSS became weaker after 20 h of UV-irradiation and heating for 20 h at 120 °C, which also revealed that the photo- and thermal-stability of the hybrid material were improved when compared to the mono-modified POSS derivative.
Some possible reasons for the enhanced luminescence properties of Tb@OBAPOSS may be explained as follows: the grafting of 8-fold BA moieties on the periphery of the POSS core can effectively increase the lanthanide concentration doped in the hybrid material. Moreover, the rigid inorganic silica-core provides high mechanical and physical properties. In addition, the 3D divergent configuration of OBAPOSS can effectively ensure the uniform distribution of lanthanide in the hybrid material (Scheme 2), which prevents the self-quenching of the lanthanide complex caused by aggregation phenomena at high concentrations.5
Experimental section
Material characterization
Amino-propyltriethoxysilane (98%, Aldrich), 4-carboxybenzaldehyde and T-heptasiloxane-triol (97%, Aldrich) were used as received. EuCl3·6H2O and TbCl3·6H2O were obtained by dissolving Eu2O3 and Tb4O7 into concentrated hydrochloric acid (37%), respectively. The excess of the acid was removed by the addition of ethanol followed by evaporation, and this process was repeated several times. Samples for thermogravimetric analysis (TGA) were transferred to open platinum crucibles and analyzed using a TA Instrument model SDT-TG Q 600 system at a heating rate of 10 °C min−1. Solid-state 29Si MAS NMR spectroscopy was performed with a Bruker Avance-500 NMR spectrometer operating at resonance frequencies of 99 MHz under a magnetic field strength of 9.4 T. FT-IR spectra were obtained on a Bruker Tensor 27 infrared spectrophotometer with a disc of KBr from 4000 to 400 cm−1 at a resolution of 4 cm−1. X-ray diffraction patterns were obtained on a Rigaku-Dmax 2500 diffractometer using Cu Kα1 radiation. SEM images were obtained using a F-SEM (Hitachi S-4300) at an acceleration voltage of 10 kV. The steady-state luminescence spectra and lifetime measurements were measured on an Edinburgh Instrument FS920P near-infrared spectrometer, with a 450 W xenon lamp as the steady-state excitation source, a double excitation monochromator (1800 lines per mm), an emission monochromator (600 lines per mm) and a semiconductor cooled Hamamatsu RMP928 photomultiplier tube.Conclusions
In summary, a novel organic–inorganic hybrid material Tb@OBAPOSS was prepared via modification at each silicon vertex of the POSS core, followed by complexation with Tb3+. This hybrid exhibits remarkable luminescent enhancement when compared to the mono-modified POSS derivative and individual rare earth complex. In particular, improvements in the photo- and thermal-stability of the luminescent hybrid material were also achieved by the combination of POSS and the lanthanide complex. On the one hand, modification at each silicon vertex in the POSS core effectively increased the lanthanide concentration doped in the hybrid material and the 3D divergent configuration of OBAPOSS ensured the uniform distribution of lanthanide in the hybrid material, which prevents the aggregation of lanthanide complex at high concentrations. On the other hand, the rigid inorganic silica-core supplied improved mechanical and physical properties. The unique luminescence properties of the hybrid material, together with its good stability and processability, make it an ideal candidate for a wide range of applications such as materials for flat-panel displays, luminescent probes in bioassays, UV sensors and as laser materials40,41 and optoelectronic devices.11,42Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (21171046, 21271060, and 21236001), the Tianjin Natural Science Foundation (13JCYBJC18400), the Natural Science Foundation of Hebei Province (No. B2013202243), and the Educational Committee of Hebei Province (2011141, LJRC021).References
- Y. S. Narayana, S. Basak, M. Baumgarten, K. Müllen and R. Chandrasekar, Adv. Funct. Mater., 2013, 23, 5875–5880 CrossRef CAS.
- H. Zhang, X. Shan, L. Zhou, P. Lin, R. Li, E. Ma, X. Guo and S. Du, J. Mater. Chem. C, 2013, 1, 888–891 RSC.
- Q. Dai, M. E. Foley, C. J. Breshike, A. Lita and G. F. Strouse, J. Am. Chem. Soc., 2011, 133, 15475–15486 CrossRef CAS PubMed.
- Y. Yue, Y. Liang, H. Wang, L. Feng, S. Feng and H. Lu, Photochem. Photobiol., 2013, 89, 5–13 CrossRef CAS PubMed.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- J. Feng and H. Zhang, Chem. Soc. Rev., 2013, 42, 387–410 RSC.
- Y.-L. Hou, H. Xu, R.-R. Cheng and B. Zhao, Chem. Commun., 2015, 51, 6769–6772 RSC.
- H. Xu, C. S. Cao and B. Zhao, Chem. Commun., 2015, 51, 10280–10283 RSC.
- P.-F. Shi, H.-C. Hu, Z.-Y. Zhang, G. Xiong and B. Zhao, Chem. Commun., 2015, 51, 3985–3988 RSC.
- H. Xu, H. C. Hu, C. S. Cao and B. Zhao, Inorg. Chem., 2015, 54, 4585–4587 CrossRef CAS PubMed.
- T. Wang, P. Li and H. Li, ACS Appl. Mater. Interfaces, 2014, 6, 12915–12921 CAS.
- H. Li, N. Lin, Y. Wang, Y. Feng, Q. Gan, H. Zhang, Q. Dong and Y. Chen, Eur. J. Inorg. Chem., 2009, 4, 519–523 CrossRef.
- V. Bekiari and P. Lianos, Adv. Mater., 1998, 10, 1455–1458 CrossRef CAS.
- Y. Wang, H. Li, Y. Feng, H. Zhang, G. Calzaferri and T. Ren, Angew. Chem., Int. Ed., 2010, 49, 1434–1438 CrossRef CAS PubMed.
- P. Cao, H. Li, P. Zhang and G. A. Calzaferri, Langmuir, 2011, 27, 12614–12620 CrossRef CAS PubMed.
- D. Wang, H. Wang and H. Li, ACS Appl. Mater. Interfaces, 2013, 5, 6268–6275 CAS.
- J. B. Lamture, Z. H. Zhou, A. S. Kumar and T. G. Wensel, Inorg. Chem., 1995, 34, 864–869 CrossRef CAS.
- H. Shou, J. Ye and Q. Yu, J. Lumin., 1988, 42, 29–34 CrossRef CAS.
- L. Hong, Z. Zhang, Y. Zhang and W. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2669–2683 CrossRef CAS.
- Y.-P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS PubMed.
- V. Agranovich, Y. N. Gartstein and M. Litinskaya, Chem. Rev., 2011, 111, 5179–5214 CrossRef CAS PubMed.
- J. Sun, Y. Chen, L. Zhao, Y. Chen, D. Qi, K. M. Choi, D. S. Shin and J. Jiang, Chem. – Eur. J., 2013, 19, 12613–12618 CrossRef CAS PubMed.
- Y. Kim, K. Koh, M. F. Roll, R. M. Laine and A. J. Matzger, Macromol., 2010, 43, 6995–7000 CrossRef CAS.
- M. Seino, W. Wang, J. E. Lofgreen, D. P. Puzzo, T. Manabe and G. A. Ozin, J. Am. Chem. Soc., 2011, 133, 18082–18085 CrossRef CAS PubMed.
- L. Zhang, J. Liu, J. Yang, Q. Yang and C. Li, Chem. – Asian J., 2008, 3, 1842–1849 CrossRef CAS PubMed.
- M. Janssen, J. Wilting, C. Müller and D. Vogt, Angew. Chem., 2010, 122, 7904–7907 CrossRef.
- F. J. Feher, J. Am. Chem. Soc., 1986, 108, 3850–3852 CrossRef CAS.
- F. J. Feher, D. A. Newman and J. F. Walzer, J. Am. Chem. Soc., 1989, 111, 1741–1748 CrossRef CAS.
- O. Türünç and M. A. Meier, Green Chem., 2011, 13, 314–320 RSC.
- V. K. Daga, E. R. Anderson, S. P. Gido and J. J. Watkins, Macromolecules, 2011, 44, 6793–6799 CrossRef CAS.
- J. Choi, J. Harcup, A. F. Yee, Q. Zhu and R. M. Laine, J. Am. Chem. Soc., 2001, 123, 11420–11430 CrossRef CAS PubMed.
- Y. Wang, A. Vaneski, H. Yang, S. Gupta, F. Hetsch, S. V. Kershaw, W. Y. Teoh, H. Li and A. L. Rogach, J. Phys. Chem. C, 2013, 117, 1857–1862 CAS.
- Y.-C. Lin and S.-W. Kuo, Polym. Chem., 2012, 3, 882–891 RSC.
- X. Chen, P. Zhang, T. Wang and H. Li, Chem. – Eur. J., 2014, 20, 2551–2556 CrossRef CAS PubMed.
- L. Li, S. Feng and H. Liu, RSC Adv., 2014, 4, 39132–39139 RSC.
- V. Ervithayasuporn, X. Wang, B. Gacal, B. N. Gacal, Y. Yagci and Y. Kawakami, J. Organomet. Chem., 2011, 696, 2193–2198 CrossRef CAS.
- P. Nockemann, E. Beurer, K. Driesen, R. Van Deun, K. Van Hecke, L. Van Meervelt and K. Binnemans, Chem. Commun., 2005, 4354–4356 RSC.
- Y. Ding, Y. Wang, H. Li, Z. Duan, H. Zhang and Y. Zheng, J. Mater. Chem., 2011, 21, 14755–14759 RSC.
- T. Pagnot, P. Audebert and G. Tribillon, Chem. Phys. Lett., 2000, 322, 572–578 CrossRef CAS.
- M. H. Werts, R. T. Jukes and J. W. Verhoeven, Phys. Chem. Chem. Phys., 2002, 4, 1542–1548 RSC.
- A. Beeby, S. W. Botchway, I. M. Clarkson, S. Faulkner, A. W. Parker, D. Parker and J. G. Williams, J. Photochem. Photobiol., B, 2000, 57, 83–89 CrossRef CAS.
- M. Lezhnina, M. Bentlage and U. Kynast, Opt. Mater., 2011, 33, 1471–1475 CrossRef CAS.
- C. Ni, G. Ni, L. Zhang, J. Mi, B. Yao and C. Zhu, J. Colloid Interface Sci., 2011, 362, 94–99 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ce01664a |
| This journal is © The Royal Society of Chemistry 2016 |