Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boronate based fluorescence (ESIPT) probe for peroxynitrite†
Adam C.
Sedgwick
a,
Xiaolong
Sun
a,
Gyoungmi
Kim
b,
Juyoung
Yoon
*b,
Steven D.
Bull
*a and
Tony D.
James
 *a
*a
aDepartment of Chemistry, University of Bath, BA2 7AY, UK. E-mail: T.D.James@bath.ac.uk
bDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr
First published on 19th September 2016
Abstract
A simple probe for the detection of peroxynitrite was developed incorporating a benzyl boronic ester “protecting” unit. The “protecting” unit of the probe is removed by peroxynitrite to “turn-on” ESIPT fluorescence (4.5 fold enhancement). Furthermore, the probe was cell permeable and was used in cell imaging experiments showing an off–on response towards peroxynitrite, in HeLa and RAW 264.7 cells.
Peroxynitrite (ONOO−) is a short lived reactive nitrogen species (RNS)1,2 with a half-life of ∼10–20 ms known to be a key pathological intermediate in a wide range of diseases, including inflammatory, ischemia–reperfusion and neurodegenerative diseases.3–5 Peroxynitrite behaves as a strong oxidant and nitrating agent towards a wide range of biological targets such as lipids, proteins, and DNA.6 Therefore the development of peroxynitrite selective fluorescent molecular probes is highly desirable.7–15 However, current commercial peroxynitrite probes including aminophenyl fluorescein (APF) and hydroxyphenyl fluorescein (HPF) lack selectivity and react with other ROS.16
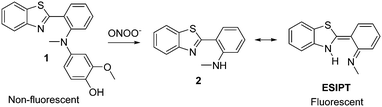
Recently Li et al. developed a “turn-on” fluorescent probe 1 for tracking the in situ generation of peroxynitrite in cells and mice using ischemia-induced neurovascular damage.6 Benzothiazole was shown to be a good fluorescent probe with high “turn-on” (600 fold), high selectivity towards peroxynitrite and rapid transport across the blood brain barrier. As shown in Scheme 1 in its normal form 2 is weakly fluorescent but when excited it can isomerise via excited state intramolecular proton transfer (ESIPT), resulting in a large increase in fluorescence.
Probe 1 was designed to contain a saturated C–N bond thus blocking the ESIPT process, however, on reaction with peroxynitrite the p-hydroxyaniline group undergoes oxidative cleavage to form benzoquinone and generates the highly fluorescent N-methyl-benzothiazole proton donor (Scheme 1).
We realised that the fluorescent N-methyl-benzothiazole fragment 2 provides a new core on which to develop a range of selective probes. Our group is particularly interested in incorporating benzyl boronic ester units, which have been elegantly employed by Chang and Shabat,17–19 this “protecting group” is oxidatively cleaved by hydrogen peroxide to generate highly fluorescent products.
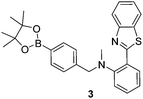
Similarly, it has been shown that boronates/boronic acids react more rapidly (milliseconds) with peroxynitrite when compared to hydrogen peroxide where the oxidation process can take several hours.20 Therefore, we decided to incorporate the Shabat “protecting group” with the fluorescent N-methyl-benzothiazole core in order to develop peroxynitrite sensor 3.
Probe 3 was synthesised over three steps through the reaction of 2-aminothiophenol and isatoic anhydride to afford 2-(benzo[d]thiazol-2-yl) aniline in 72% yield. A one pot reductive amination was then carried out using sodium triacetoxyborohydride and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde in 78% yield. The intermediate formed was found to contain a particularly unreactive aniline towards a number of reagents including NaH, consequently an excess of reagents was required, therefore, 15 equivalents of AcOH and 10 equivalents of formaldehyde were used to afford probe 3 in 14% yield (Scheme S1, ESI†).
We subsequently evaluated the fluorescence behaviour of probe 3, in pH 8.2 buffer solution [52 wt% methanol].21 Probe 3 produced an up to 4.5 fold fluorescence “turn on” in the presence of low concentrations of peroxynitrite (0–10 μM) (Fig. 1).
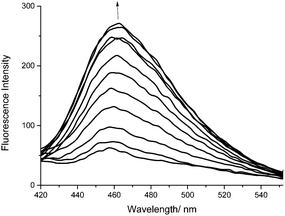 | ||
| Fig. 1 Fluorescence spectra of probe 3 (0.25 μM) with the addition of peroxynitrite (0–10 μM), λex 400 nm in pH 8.2 buffer solution [52 wt% methanol]. | ||
Subsequently, we evaluated the selectivity of probe 3 towards other ROS/RNS. As expected, ClO− (100 μM) led to a decrease in fluorescence intensity (0.71) due to its strong oxidising ability slowly destroying probe 3. While, as reported by Sikora et al., H2O2 led to a small fluorescence enhancement at 100 μM. Addition of 10 mM H2O2 is required to produce a significant fluorescence response (Fig S3 and S4, ESI†), clearly demonstrating the greater reactivity of boronic acids/esters towards peroxynitrite (Fig. 2).
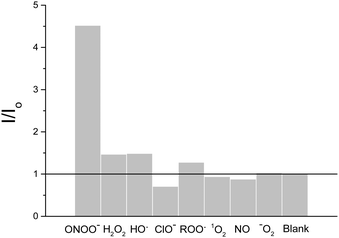 | ||
| Fig. 2 Selectivity of probe 3 (0.25 μM), 10 μM peroxynitrite. All other ROS 100 μM. H2O2, ClO− and ROO; incubated for 30 min, λex 400 nm/λem 461 nm in pH 8.2 buffer solution [52 wt% methanol]. | ||
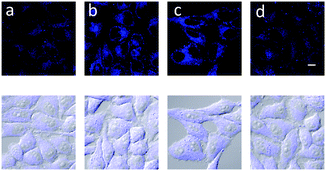
Having determined the selectivity of probe 3, we then evaluated its ability to visualise endogenous and exogenous peroxynitrite using cell imaging experiments. HeLa cells were incubated with probe 3 (20 μM) for 30 minutes and washed with Dulbecco's phosphate-buffered saline (DPBS). The cells were then observed using a confocal laser microscope excitation λ 405 nm, emission λ 430–455 nm. As shown in Fig. 3 probe 3 can penetrate live cell membranes and provide a clear “turn on” response in the presence of various concentrations of peroxynitrite added exogenously. No “turn on” was observed when the cells were pretreated with the peroxynitrite scavenger ebselen (Fig. 3).
To detect peroxynitrite endogenously, RAW 264.7 cells were used and the immune reaction was induced using 1 μg mL−1 and 50 ng mL−1 LPS, IFN-γ. As displayed above in Fig. 4, probe 3 was shown to detect peroxynitrite endogenously. Probe 3 was also evaluated at longer wavelengths in cells λex 473 nm and λem 490–590 nm (Fig. S7, ESI†).
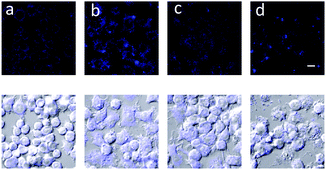 | ||
| Fig. 4 Detection of peroxynitrite made by immune reaction in the macrophage. RAW 264.7 cells were treated with (a) no, (b) 1 μg mL−1 LPS 16 h, 50 ng mL−1 interferon-γ 4 h, (c), LPS, IFN-γ + 100 μM ebselen and (d) LPS, IFN-γ + 100 μM uric acid. The cells were stained with 20 μM probe 3 for 30 min and washed with DPBS and imaged by confocal microscopy. λex 405 nm/λem 430–455 nm. Scale bar: 10 μm.22–24 | ||
In conclusion we have developed a reaction based fluorescent probe that can be used to detect peroxynitrite at low concentrations allowing the detection in cells. Before exposure to ROS, probe 3 has a low fluorescence intensity because the ESIPT process is not possible. However, the fluorescence is enhanced once the boronic ester “protecting group” is removed selectively by exposure to peroxynitrite. We have demonstrated that N-methyl-benzothiazole core unit 2 can be used to develop probes with applications in exploring the pathological effect of peroxynitrite. Furthermore, probe 3 has a very important advantage over probe 1 since it could be easily developed into a theranostic agent towards tumour cells. This approach was recently demonstrated by Kim et al. who have used the boronic “protecting group” to weaponize a probe by linking with the anticancer prodrug 5′-deoxy-5-fluorouridine to the boronic acid group.25
ACS, XS, SDB, and TDJ would like to thank the University of Bath for support. ACS thanks the EPSRC for a studentship. JY thanks the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2012R1A3A2048814). All data created during this research are openly available from the University of Bath data archive at http://doi.org/10.15125/BATH-00252.
Notes and references
- J. S. Beckman, T. W. Beckman, J. Chen, P. A. Marshall and B. A. Freeman, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 1620–1624 CrossRef CAS.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed.
- H. Ischiropoulos and J. S. Beckman, J. Clin. Invest., 2003, 111, 163–169 CrossRef CAS PubMed.
- F. Torreilles, S. Salman-Tabcheh, M.-C. Guérin and J. Torreilles, Brain Res. Rev., 1999, 30, 153–163 CrossRef CAS PubMed.
- C. S. Wilcox and A. Pearlman, Pharmacol. Rev., 2008, 60, 418–469 CrossRef CAS PubMed.
- X. Li, R.-R. Tao, L.-J. Hong, J. Cheng, Q. Jiang, Y.-M. Lu, M.-H. Liao, W.-F. Ye, N.-N. Lu, F. Han, Y.-Z. Hu and Y.-H. Hu, J. Am. Chem. Soc., 2015, 137, 12296–12303 CrossRef CAS PubMed.
- F. Yu, P. Li, G. Li, G. Zhao, T. Chu and K. Han, J. Am. Chem. Soc., 2011, 133, 11030–11033 CrossRef CAS PubMed.
- F. Yu, P. Li, B. Wang and K. Han, J. Am. Chem. Soc., 2013, 135, 7674–7680 CrossRef CAS PubMed.
- Z. Lou, P. Li and K. Han, Acc. Chem. Res., 2015, 48, 1358–1368 CrossRef CAS PubMed.
- X. Zhou, Y. Kwon, G. Kim, J.-H. Ryu and J. Yoon, Biosens. Bioelectron., 2015, 64, 285–291 CrossRef CAS PubMed.
- X. Chen, F. Wang, J. Y. Hyun, T. Wei, J. Qiang, X. Ren, I. Shin and J. Yoon, Chem. Soc. Rev., 2016, 45, 2976–3016 RSC.
- X. Sun, K. Lacina, E. C. Ramsamy, S. E. Flower, J. S. Fossey, X. Qian, E. V. Anslyn, S. D. Bull and T. D. James, Chem. Sci., 2015, 6, 2963–2967 RSC.
- X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, Chem. Sci., 2014, 5, 3368–3373 RSC.
- T. Peng, N.-K. Wong, X. Chen, Y.-K. Chan, D. H.-H. Ho, Z. Sun, J. J. Hu, J. Shen, H. El-Nezami and D. Yang, J. Am. Chem. Soc., 2014, 136, 11728 CrossRef CAS PubMed.
- Z.-J. Chen, W. Ren, Q. E. Wright and H.-W. Ai, J. Am. Chem. Soc., 2013, 135, 14940–14943 CrossRef CAS PubMed.
- K. Setsukinai, Y. Urano, K. Kakinuma, H. J. Majima and T. Nagano, J. Biol. Chem., 2003, 278, 3170–3175 CrossRef CAS PubMed.
- O. Redy-Keisar, E. Kisin-Finfer, S. Ferber, R. Satchi-Fainaro and D. Shabat, Nat. Protoc., 2014, 9, 27–36 CrossRef CAS PubMed.
- A. R. Lippert, G. C. V. De Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed.
- M. E. Roth, O. Green, S. Gnaim and D. Shabat, Chem. Rev., 2016, 116, 1309–1352 CrossRef CAS PubMed.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef CAS PubMed.
- D. D. Perrin and B. Dempsey, Buffers for pH and Metal Ion Control, Chapman and Hall, London, 1974 Search PubMed.
- Z. Balafanova, R. Bolli, J. Zhang, Y. Zheng, J. M. Pass, A. Bhatnagar, X.-L. Tang, O. Wang, E. Cardwell and P. Ping, J. Biol. Chem., 2002, 277, 15021 CrossRef CAS PubMed.
- S. Csaba, I. Harry and R. Rafael, Nat. Rev. Drug Discovery, 2007, 6, 662 CrossRef PubMed.
- R. B. Kean, S. V. Spitsin, T. Mikheeva, G. S. Scott and D. C. Hooper, J. Immunol., 2000, 165, 6511 CrossRef CAS.
- R. Kumar, J. Han, H.-J. Lim, W. X. Ren, J.-Y. Lim, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 17836 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc06829d |
| This journal is © The Royal Society of Chemistry 2016 |