Open Access Article
Open Access Article{Tc(NO)(Cp)(PPh3)}+ – a novel technetium(I) core†
J.
Ackermann
,
A.
Hagenbach
and
U.
Abram
*
Freie Universität Berlin, Institute of Chemistry and Biochemistry, Fabeckstr. 34/36, D-14195 Berlin, Germany. E-mail: Ulrich.Abram@fu-berlin.de
First published on 22nd July 2016
Abstract
Reactions between [TcI(NO)X2(PPh3)2(CH3CN)] complexes (X = Cl, Br) and KCp form the pseudotetrahedral organotechnetium compounds [TcI(NO)(Cp)(PPh3)X]. The halide ligands can readily be replaced by other halides or organometallic ligands giving access to a novel family of technetium(I) compounds with the robust {Tc(NO)(Cp)(PPh3)}+ core.
There is considerable interest in organotechnetium compounds as potential building blocks in bioconjugates for nuclear medical imaging.1–7 Many such approaches use the tricarbonyl core {99mTc(CO)3}+, which can readily be accessed using the IsoLink kit and is used for the complexation of a large variety of preferably tripodal ligand systems.7–10 Also promising attempts with cyclopentadienyl (Cp−) derivatives have been described recently.11–15 Nevertheless, there is an enormous lack of structural information about organotechnetium compounds, since the corresponding rhenium complexes are frequently only insufficient models, particularly for non-tricarbonyl compounds and when redox processes are involved in the synthetic procedures.
Complexes of technetium with Cp− derivatives are rare and only 14 of them have hitherto been characterized unambiguously by X-ray crystallography,16–26 while more than 800 entries for the corresponding rhenium compounds can be found in the CSD database.27 This lack of knowledge has not only to do with the radioactivity of technetium, but also with the fact that the 99Tc analogues of many precursors used for the synthesis of organorhenium compounds are difficult to access, less stable and/or volatile. At first glance, it is particularly surprising that the chemistry of nitrosyltechnetium complexes with cyclopentadienyl ligands has not yet been considered so far, since there exist a vast number of papers, which describe the fascinating chemistry of compounds of the general composition [Re(NO)(Cp)(PPh3)X]0,+, where X can be almost any monodentate ligand or organometallic building block.28–34 A closer look at this point, however, shows that the synthetic approach to the {Re(NO)(Cp)(PPh3)}+ core, particularly to the excellent precursor molecule [Re(NO)(Cp)(PPh3)Cl], cannot readily be adopted for the chemistry of technetium. It commonly starts from carbonyl species with subsequent reduction of a CO ligand to formyl and methyl and the final replacement of the methyl unit by Cl−. Such a procedure is still a challenge to be done with 99Tc, even when an improved synthesis for (NEt4)2[99TcCl3(CO)3] has been reported recently.35
In the present communication we describe a convenient synthesis of [Tc(NO)(Cp)(PPh3)Cl] (1) and some ongoing reactions (Fig. 1), which qualify this compound as a suitable precursor for the further exploration of the chemistry of the {Tc(NO)(Cp)(PPh3)}+ core.
The molecular structure of 2 (Fig. 2) reveals the expected pseudotetrahedral structure with a η5-bonded cyclopentadienyl ring. It is similar to that of the analogous rhenium complex [Re(NO)(MeCp)(PPh3)I].38 Crystal structure data of the corresponding [Re(NO)(Cp)(PPh3)X] complexes (X = Cl, Br, I) are unfortunately not available. The measured bond lengths and angles are unexceptional and confirm the results derived from the spectroscopic data. The nitrosyl ligand is almost linearly coordinated and acts as a 3-electron donor as has been observed in all hitherto studied Tc(NO) complexes.36,37,39–46
 | ||
| Fig. 2 Structure of 2. Selected bond lengths: Tc–N 1.869(9), N–O1 1.03(1), Tc–P 2.369(8), Tc–Br 2.471(9), and Tc–Cp(centroid) 1.937(2) Å. | ||
Compound 1 has been used for some fundamental reactions in order to review its suitability as a precursor for the synthesis of more organotechnetium compounds and to find out restricting conditions for such ongoing reactions. A summary of the first results is given in Fig. 1. It becomes evident that the Cl− ligand can readily be replaced under conservation of the {Tc(NO)(Cp)(PPh3)}+ core. Reactions with HBr or Me3SiBr give the bromide derivative, which can also be prepared directly from [TcI(NO)Br2(PPh3)2(CH3CN)].
Bubbling CO gas through a solution of 1 and addition of Ag(BF4) results in the formation of red crystals of [Tc(NO)(Cp)(PPh3)(CO)](BF4) (3). The compound is soluble in common organic solvents and stable in air. Its IR spectrum shows the ν(CO) band at 2037 cm−1, while the ν(NO) frequency appears at 1776 cm−1. The latter, relatively high value reflects a lower degree of backdonation to the nitrosyl ligand in 3 compared to 1 or 2. A similar trend of the ν(NO) bands is also observed for the analogous rhenium compounds. A comparison is given in the ESI† (Table S9).
An ellipsoid plot of the structure of the complex cation of 3 is shown in Fig. 3. Bond lengths and angles of the remaining coordination sphere are not significantly influenced by the replacement of Cl− by CO.
 | ||
| Fig. 3 Structure of the cation of 3. Selected bond lengths: Tc–N 1.679(8), N–O1 1.155(3), Tc–P 2.369(8), Tc–C(carbonyl) 1.832(2), C–O2 1.133(3), and Tc–Cp(centroid) 1.944(1) Å. | ||
The reaction of 1 with phenyl magnesium bromide gives the phenyl derivative 4 in good yields. The synthetic procedure was adopted from that of the analogous Re compound.47 Also the solid state structure of 4 corresponds to that of [Re(NO)(Cp)(PPh3)(Ph)], which means that both compounds crystallize in the polar space group P21 with only one enantiomer of the chiral complex in the crystals, while 2 and 3 crystallize in centrosymmetric space groups as racemates. An ellipsoid plot of 4 is shown in Fig. 4. The Tc–phenyl bond length in 4 of 2.149(2) Å is in the magnitude of the few other hitherto studied Tc–aryl bonds.48 It is interesting to note that the orientation of the phenyl ligand is almost identical to that in the analogous rhenium complex with angles of 69.28(7)° and −16.51(5)° between the least-squares plane of the phenyl ring and the Tc–P and Tc–N bonds. In such rotamers, the hydrogen atoms attached to C1 and C42 come close together (2.89 Å in the rhenium complex and 2.398(1) Å in 4), see Fig. S3 (ESI†). In solution, there is no evidence for such interactions as is reflected by the NMR spectra of the compounds. That of 4 shows one sharp singlet for the cyclopentadienyl protons at 5.10 ppm and an unexceptional coupling pattern for the phenyl ligand, the signals of which are well separated from those of PPh3.
 | ||
| Fig. 4 Structure of 4. Selected bond lengths: Tc–N 1.758(2), N–O 1.198(2), Tc–P 2.357(1), Tc–C 2.47(1), Tc–C(phenyl) 2.149(2), and Tc–Cp(centroid) 1.972(1) Å. | ||
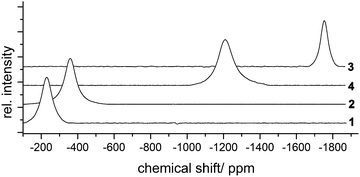
99Tc-NMR spectroscopy is a helpful tool for the characterization particularly of Tc(VII) and Tc(I) compounds. The nucleus has a high NMR sensitivity, which is relative to the 1H resonance, 0.275,49 but the signals frequently show large line-widths due to the noticeable quadrupole moment of Q = −0.19(5) × 10−28 m2.50 This is also the case for the pseudotetrahedral compounds of the present study, which all have four different ligands each and show 99Tc-NMR signals with line-widths between 3900 and 8800 Hz. Nevertheless, the spectra can readily be recorded within a reasonable time. The signals appear between −231 and −1753 ppm (Fig. 5), which is typical for Tc(I) compounds.51 Such a large range of chemical shifts for structurally closely related compounds indicates a significant influence of the Cl−, Br−, Ph− and CO ligands on the electronic situation of the technetium atoms. For a more quantitative discussion of this point, however, more representatives are required.
There is an ongoing discussion about the existence of the Tc(VII) compound [TcO3(Cp)].52–54 All attempts to isolate a molecular compound analogous to [ReO3(Cp)], e.g. by the oxidation of [Tc(CO)3(Cp)] with H2O2 or other oxidants, were hitherto without success,19,54,55 and there is some discussion about the general non-existence of this technetium compound.54 This stimulated us to attempt oxidation reactions starting from the novel Cp compounds. Reactions of 1 or 2 with H2O2 in acetonitrile directly give pertechnetate. The starting materials as well as the product are well detected by 99Tc-NMR spectroscopy, and we could not find any evidence for the formation of [TcO3(Cp)] or other TcI or TcVII intermediates during the corresponding NMR experiment.
The lability of the Cp− ligand in the title complexes upon oxidation is also shown during another experiment: the oxidation of 2 with equivalent amounts of bromine, which gives the technetium(II) complex [Tc(NO)Br3(OPPh3)2] (5) as the exclusive product, while similar reactions with [Re(Cp)(CO)3] or [Tc(Cp*)(CO)3] gave [MIII(Cp*)Br2(CO)2] (M = Tc, Re) and [TcIII(Cp*)Br(CO)3]+, respectively.19,55 Compound 5 (for an ellipsoid plot see Fig. S4 of the ESI†) is a hitherto unknown member of the family of octahedral phosphine/phosphine oxide nitrosyltechnetium(II) complexes, with one of the OPPh3 ligands being coordinated equatorially, while the second one is in the trans position to NO. The Tc–O bond to the latter one is slightly lengthened indicating a considerable trans influence of the nitrosyl ligand. While the room temperature EPR spectrum of the Tc(II) compounds shows the expected 10-line hyperfine structures (hfs) due to the interaction of the unpaired electron with the nuclear spin of 99Tc (I = 9/2), the anisotropic EPR spectrum of a frozen solution of the compound (Fig. 6) has axially-symmetric features with resolved 99Tc hfs patterns in the parallel and perpendicular parts.
 | ||
| Fig. 6 Frozen solution X-band EPR spectra of 5 in CH2Cl2 at 77 K (g∥ = 2.042(1), ATC∥ = 237.3 (±1.0) × 10−4 cm−1, g⊥ = 2.059(1), ATc∥ = 104.21 (±1.0) × 10−4 cm−1). | ||
In conclusion, previously unreported technetium Cp complexes have been synthesized, closing a gap in the organometallic chemistry of the homologous elements technetium and rhenium. The ready synthesis of the parent complexes 1 and 2 recommends them as precursors for ongoing work in order to explore the full synthetic potential of the {Tc(NO)(Cp)(PPh3)}+ core. Such experiments will be the subject of further studies in our laboratory, also including attempts to find an approach to related 99mTc compounds for nuclear medical procedures.
References
- R. Alberto and U. Abram, in Handbook of Nuclear Chemistry, ed. A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas and F. Rösch, Springer, 2011, vol. 4, pp. 2073–2120 Search PubMed.
- P. J. Blower and S. Prakash, in Perspectives on Bioinorganic Chemistry, ed. R. W. Hay, J. R. Dilworth and K. B. Nolan, JAI Press Inc., 1999, vol. 4, pp. 91–143 Search PubMed.
- S. Bhattacharyya and M. Dixit, Dalton Trans., 2011, 40, 6112 RSC.
- E. W. Price and C. Orvig, Chem. Soc. Rev., 2014, 43, 260 RSC.
- C. S. Cutler, H. M. Hennkens, N. Sisay, S. Huclier-Markai and S. S. Jurisson, Chem. Rev., 2013, 113, 858 CrossRef CAS PubMed.
- U. Abram and R. Alberto, J. Braz. Chem. Soc., 2006, 17, 1486 CrossRef CAS.
- G. R. Morais, A. Paulo and I. Santos, Organometallics, 2012, 31, 5693 CrossRef CAS.
- R. Alberto, R. Schibli, R. Waibel, U. Abram and A. P. Schubiger, Coord. Chem. Rev., 1999, 190–192, 901 CrossRef CAS.
- R. Alberto, Organometallic Radiopharmaceutical, in Topics in Organometallic Chemistry, ed. G. Jaouen and N. Metzler-Nolte, Springer, Berlin, Heidelberg, 2010, pp. 219–246 Search PubMed.
- I. Santos, A. Paulo and J. D. G. Correia, Rhenium and Technetium Complexes Anchored by Phosphines and Scorpionates, for Radiopharmaceutical Applications, in Topics in Current Chemistry, ed. W. Krause, Springer, Berlin, Heidelberg, 2005, pp. 45–84 Search PubMed.
- Y. Liu, B. Spingler, P. Schmutz and R. Alberto, J. Am. Chem. Soc., 2008, 130, 1554 CrossRef CAS PubMed.
- H. W. P. N'Dongo, P. D. Raposinho, C. Fernandes, I. Santos, D. Can, P. Schmutz, B. Spingler and R. Alberto, Nucl. Med. Biol., 2010, 37, 255 CrossRef PubMed.
- S. Sulieman, D. Can, J. Mertens, H. W. P. N'Dongo, Y. Liu, P. Schmutz, M. Bauwens, B. Spingler and R. Alberto, Organometallics, 2012, 31, 6880 CrossRef CAS.
- G. R. Morais, A. Paulo and I. Santos, Organometallics, 2012, 31, 5693 CrossRef CAS.
- X. Wang, D. Li, W. Deuther-Conrad, J. Lu, Y. Xie, B. Lia, M. Cui, J. Steinbach, P. Brust, B. Liu and H. Jia, J. Med. Chem., 2014, 57, 7113 CrossRef CAS PubMed.
- C. Apostolidis, B. Kanellakopulos, R. Maier, J. Rebizant and M. L. Ziegler, J. Organomet. Chem., 1990, 396, 315 CrossRef CAS.
- K. Raptis, B. Kanellakopulos, B. Nuber and M. L. Ziegler, J. Organomet. Chem., 1991, 405, 323 CrossRef CAS.
- K. Raptis, E. Dornberger, B. Kanellakopulos, B. Nuber and M. L. Ziegler, J. Organomet. Chem., 1991, 408, 61 CrossRef CAS.
- H. H. K. Castro, A. Meetsma, J. H. Teuben, W. Vaalsburg, K. Panek and G. Ensing, J. Organomet. Chem., 1991, 410, 63 CrossRef CAS.
- C. Apostolidis, B. Kanellakopulos, R. Maier, J. Rebizant and M. L. Ziegler, J. Organomet. Chem., 1991, 411, 171 CrossRef CAS.
- E. O. Fischer, C. Apostolidis, E. Dornberger, A. C. Filippou, B. Kanellakopulos, B. Lungwitz, J. Müller, B. Powietzka, J. Rebizant and W. Roth, Z. Naturforsch., 1995, B50, 1382 Search PubMed.
- J. E. Joachim, C. Apostolidis, B. Kanellakopulos, D. Meyer, B. Nuber, K. Raptis, J. Rebizant and M. L. Ziegler, J. Organomet. Chem., 1995, 492, 199 CrossRef CAS.
- J. Bernard, K. Ortner, B. Spingler, H.-J. Pietzsch and R. Alberto, Inorg. Chem., 2003, 42, 1014 CrossRef CAS PubMed.
- S. Masi, S. Top, L. Boubekeur, G. Jaouen, S. Mundwiler, B. Spingler and R. Alberto, Eur. J. Inorg. Chem., 2004, 2013 CrossRef CAS.
- F. Zobi, B. Spingler and R. Alberto, Eur. J. Inorg. Chem., 2008, 4002 Search PubMed.
- A. K. Burrell and J. C. Bryan, Organometallics, 1992, 11, 3501 CrossRef CAS.
- Cambridge Crystallographic Data Center, Database ver. 3.27 (November, 2015).
- F. Agbossou, E. J. O_Connor, C. M. Garner, N. Quiros Mendez, J. M. Fernandez, A. T. Patton, J. A. Ramsden and J. A. Gladysz, Inorg. Synth., 1992, 29, 211 CrossRef CAS.
- S. N. Seidel, M. Prommesberger, S. Eichenseher, O. Meyer, F. Hampel and J. A. Gladysz, Inorg. Chim. Acta, 2010, 363, 533 CrossRef CAS.
- R. Dembinski, T. Lis, S. Szafert, C. L. Mayne, T. Bartik and J. A. Gladysz, J. Organomet. Chem., 1999, 578, 229 CrossRef CAS.
- N. Quiros Mendez, A. M. Arif and J. A. Gladysz, Organometallics, 1991, 10, 2199 CrossRef CAS.
- S. Eichenseher, O. Delacroix, K. Kromm, F. Hampel and J. A. Gladysz, Organometallics, 2005, 24, 245 CrossRef CAS.
- K. Kromm, F. Hampel and J. A. Gladysz, Organometallics, 2002, 21, 4264 CrossRef CAS.
- S. Legoupy, C. Crevisy, J.-C. Guillemin, R. Gree and L. Toupet, Chem. – Eur. J., 1998, 4, 2162 CrossRef CAS.
- S. Mundwiler, H. Braband, R. Alberto, D. Mcgregor, R. C. Howell and L. C. Francesconi, Inorg. Synth., 2014, 36, 149 CrossRef CAS.
- R. A. Armstrong and H. Taube, Inorg. Chem., 1976, 15, 1904 CrossRef CAS.
- S. M. Balasekaran, J. Spandl, A. Hagenbach, K. Köhler, M. Drees and U. Abram, Inorg. Chem., 2014, 53, 5117 CrossRef CAS PubMed.
- C. H. Winter, W. R. Veal, A. M. Arif and J. A. Gladysz, J. Am. Chem. Soc., 1989, 111, 4766 CrossRef CAS.
- T. Nicholson, E. Chun, A. Mahmood, P. Müller and A. Davison, Commun. Inorg. Synth., 2015, 3, 31 Search PubMed and references cited therein.
- J. Ackermann, A. Hagenbach and U. Abram, Inorg. Chim. Acta, 2014, 419, 59 CrossRef CAS.
- R. Schibli, N. Marti, P. Maurer, B. Spingler, M.-L. Lehaire, V. Gramlich and C. L. Barnes, Inorg. Chem., 2005, 44, 693 CrossRef PubMed.
- K. E. Linder, A. Davison, J. C. Dewan, C. E. Costello and S. Maleknia, Inorg. Chem., 1986, 25, 2085 CrossRef CAS.
- L. J. Radonovich and J. L. Hoard, J. Phys. Chem., 1984, 88, 6711 CrossRef CAS.
- J. Baldas, J. F. Boas, J. Bonnyman and G. A. Williams, J. Chem. Soc., Dalton Trans., 1984, 827 RSC.
- R. M. Pearlstein, W. M. Davis, A. G. Jones and A. G. Jones, Inorg. Chem., 1989, 28, 3332 CrossRef CAS.
- D. S. Brown, J. L. Newman and J. R. Thornback, Acta Crystallogr., 1988, C44, 973 CAS.
- S. K. Agbossou, G. S. Bodner, A. T. Patton and J. A. Gladysz, Organometallics, 1990, 9, 1184 CrossRef CAS.
- E. Oehlke, S. Kong, P. Arciszewski, S. Wiebalck and U. Abram, J. Am. Chem. Soc., 2012, 134, 9118 CrossRef CAS PubMed.
- R. K. Harris, NMR and the Periodic Table, Academic, London, 1978 Search PubMed.
- D. Wendtland, J. Bauche and P. Luc, J. Phys. B: At. Mol. Phys., 1979, 10, 1989 CrossRef.
- V. A. Mikhalev, Radiochemistry, 2005, 47, 319 CrossRef CAS.
- B. Kanellakopoulos, B. Nuber, K. Raptis and M. L. Ziegler, Angew. Chem., Int. Ed. Engl., 1989, 28, 1055 CrossRef.
- A. W. E. Chan, R. Hoffmann and S. Alvarez, Inorg. Chem., 1991, 30, 1086 CrossRef CAS.
- A. K. Burrell, F. A. Cotton, L. M. Daniels and V. Petricek, Inorg. Chem., 1995, 34, 4253 CrossRef CAS.
- R. Alberto, R. Schibli, A. Egli, U. Abram, S. Abram, T. A. Kaden and P. A. Schubiger, Polyhedron, 1998, 17, 1133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectroscopic data and details about the structural determinations, and tables with bond lengths and angles. CCDC 1491441–1491444. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc05811f |
| This journal is © The Royal Society of Chemistry 2016 |