Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aluminium salalens vs. salans: “Initiator Design” for the isoselective polymerisation of rac-lactide†
Paul
McKeown
ab,
Matthew G.
Davidson
b,
Gabriele
Kociok-Köhn
b and
Matthew D.
Jones
*b
aDoctoral Training Centre in Sustainable Chemical Technologies, University of Bath, Bath BA2 7AY, UK
bDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: mj205@bath.ac.uk; Fax: +44 (0) 1225 386231; Tel: +44 (0) 1225 384908
First published on 27th July 2016
Abstract
We report the rationalised design of aluminium initiators and their application for ROP of rac-lactide (rac-LA). A very minor change to the ligand backbone (imine reduction) to give secondary amines was found to have a dramatic effect on activity and selectivity with isotactic PLA being realised.
There is a significant range of aluminium initiators reported for the ROP of rac-LA to afford polylactide (PLA). Aluminium is attractive as it is a highly abundant metal. Initiators based on aluminium are often reported to exert some degree of stereocontrol over the polymerisation of rac-LA with there being cases of both isotactic and heterotactic preference in the literature,1 it is difficult to predict or rationalise the selectivity. While such polymerisations are well controlled in terms of polymer architecture and weight properties, they often suffer from slow rates with many hours to several days required in solution and even melt polymerisations. A challenge for the ROP of rac-LA is the preparation of isotactic stereoblock PLA under industrially relevant conditions and determining initiator structure activity relationships. Other metal centres that are active for the production of isotactic PLA include Zr(IV),2 Hf(IV),1l,2b,d Y(III),3 In(III),4 Zn(II)5 and lanthanides.6
One of the earliest examples of stereocontrolled PLA production was demonstrated by Spassky and co-workers.1j Using chiral binaphthyl Schiff base aluminium complexes, isotacticity resulted from the solution ROP (70 °C, toluene, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 75
[Init] = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with evidence of stereocomplexation being observed. Reaction times from 5 hours (19% conversion) up to 281 hours (98% conversion) are reported for this system. An isotactic initiator was also prepared by Feijen et al., also utilising an aluminium salen complex.1k,n In this case, the ligand backbone was trans-1,2-cyclohexyl and Pm values of 0.93 (solution, [LA]
1) with evidence of stereocomplexation being observed. Reaction times from 5 hours (19% conversion) up to 281 hours (98% conversion) are reported for this system. An isotactic initiator was also prepared by Feijen et al., also utilising an aluminium salen complex.1k,n In this case, the ligand backbone was trans-1,2-cyclohexyl and Pm values of 0.93 (solution, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 62
[Init] = 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 0.88 (melt, [LA]
1) and 0.88 (melt, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 200
[Init] = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are reported with typical reaction times given in days. Examples of relatively fast and well controlled Al(III) salen mediated polymerisations are provided by Nomura et al.1e,f In one study, the identity of two key positions upon the ligand (substituents ortho to the phenoxy and the aliphatic backbone) are varied.1f The achiral salen complexes screened generally showed isotactic preference (Pm > 0.69) and solution reactions time varied from 0.4 to 72 hours depending on the nature of the substituents. In particular, excellent stereocontrol was realised with tBuMe2Si ortho groups and this was largely maintained at high temperatures (130–180 °C, [LA]
1) are reported with typical reaction times given in days. Examples of relatively fast and well controlled Al(III) salen mediated polymerisations are provided by Nomura et al.1e,f In one study, the identity of two key positions upon the ligand (substituents ortho to the phenoxy and the aliphatic backbone) are varied.1f The achiral salen complexes screened generally showed isotactic preference (Pm > 0.69) and solution reactions time varied from 0.4 to 72 hours depending on the nature of the substituents. In particular, excellent stereocontrol was realised with tBuMe2Si ortho groups and this was largely maintained at high temperatures (130–180 °C, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 300
[Init] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), however preparation of this particular initiator requires lengthy synthesis including protection and deprotection. Gibson et al. have prepared eight salan complexes based around a N,N′-disubstituted ethylenediamine backbone.1c Depending on substituent choice, strong heterotactic or moderate isotactic preference was observed with the solution polymerisation length typically being around 24 hours (70 °C, [LA]
1), however preparation of this particular initiator requires lengthy synthesis including protection and deprotection. Gibson et al. have prepared eight salan complexes based around a N,N′-disubstituted ethylenediamine backbone.1c Depending on substituent choice, strong heterotactic or moderate isotactic preference was observed with the solution polymerisation length typically being around 24 hours (70 °C, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 100
[Init] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). More recently, Kol et al. have demonstrated enantiomerically pure salalens based upon an aminomethylpyrrolidine moiety.1h Application of these for ROP of rac-LA gave reasonable heterotacticity or gradient isotactic multiblock PLA (Pm = 0.82) in toluene (80 °C, [LA]
1). More recently, Kol et al. have demonstrated enantiomerically pure salalens based upon an aminomethylpyrrolidine moiety.1h Application of these for ROP of rac-LA gave reasonable heterotacticity or gradient isotactic multiblock PLA (Pm = 0.82) in toluene (80 °C, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Init] = 100
[Init] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
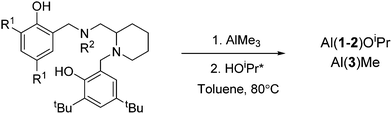
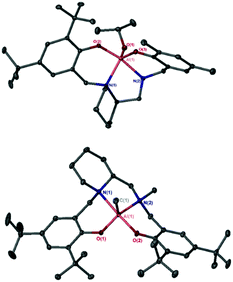
In this work, a series of related salan complexes based on 2-aminopiperidine are reported. Reduction of the imine functionality of the salalen structure afforded a secondary amine salan and subsequent methylation at this position further yields a tertiary amine based salan (Scheme 1). Important for industrial applications, the salan ligands are colourless consequently affording colourless complexes and white polymer. Ligands 1–2H2 are prepared via a simple three step synthesis which is achievable on a multigram scale within a day. All ligands have been characterised by 1H and 13C{1H} NMR spectroscopy as well as ESI-MS. The initial complexation of 1–3H2 with AlMe3 (Scheme 2) afforded complexes each with 4 distinct species in solution. It is postulated that these species are a consequence of the inherent stereochemistry of the ligand and the new stereocentres that form upon complexation; the 2nd position of the aminopiperidine ring is stereogenic and derived from a racemic reagent while the bonding of both the nitrogen centres to the metal centre also generates two further points of chirality. Thus, there are potential diastereomers present in solution. A similar observation was made for Al(A–B)Me for which there are two stereocentres on complexation and two species observed in solution.7 Further to the previous work, the Me has been exchanged with –OBn to form Al(A)OBn which was characterised as a single diastereomer by 1H NMR analysis. Solid state analysis shows the wrapping of the imino ligand around the aluminium centre to be analogous to the Al(A)Me complex with the trigonal bipyramidal geometry maintained (τ = 0.72), (Scheme 3, Table 1). For Al(1/2)Me, no suitable crystals were generated for X-ray crystallography, however, for Al(3)Me, a solid state structure was obtained and this revealed the complex structure to be similar to that of the parent imine complex with a pentacoordinate aluminium centre (τ = 0.64).
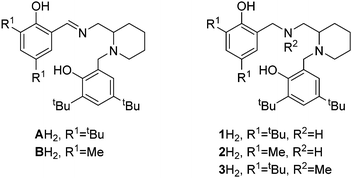 | ||
| Scheme 1 Previously reported salalen ligands7 (A/BH2) and salan ligands (1–3H2) used in this study. | ||
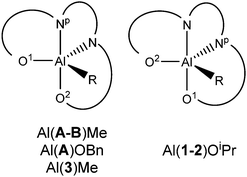 | ||
| Scheme 3 Trigonal bipyramidal isomers observed for Al(III) complexes. Np denotes the nitrogen within the piperidine ring. | ||
| Al(A)OBn | Al(1)OiPr | Al(2)OiPr | Al(3)Me | |
|---|---|---|---|---|
| a Angles used to determine τ. | ||||
| τ | 0.72 | 0.77 | 0.78 | 0.64 |
| Al–O(1) | 1.7585(10) | 1.7991(8) | 1.7955(9) | 1.7633(10) |
| Al–O(2) | 1.8262(10) | 1.7730(8) | 1.7644(9) | 1.8024(10) |
| Al–Np(1) | 2.1386(12) | 2.0672(10) | 2.0622(10) | 2.2834(12) |
| Al–N(2) | 1.9670(12) | 2.1072(10) | 2.1122(10) | 2.0786(12) |
| Al–R | 1.7356(10) | 1.7400(9) | 1.7418(9) | 1.9765(15) |
| O(1)–Al–O(2) | 95.30(5) | 94.55(4) | 96.12(4) | 91.39(5) |
| O(1)–Al–N(2) | 117.42(5) | 170.31(4)a | 169.19(4)a | 125.09(5)a |
| O(2)–Al–Np(1) | 168.02(5)a | 117.49(4) | 113.74(4) | 163.28(5)a |
| O(1)–Al–R | 124.59(5)a | 102.45(4) | 101.55(4) | 122.02(6) |
| O(2)–Al–R | 97.48(5) | 124.16(4)a | 120.53(4) | 102.08(6) |
| Np(1)–Al–R | 87.48(5) | 115.37(4) | 122.62(4)a | 93.19(6) |
| N(2)–Al–R | 117.42(5) | 82.35(4) | 82.13(4) | 115.56(6) |
The exchange of the aluminium methyl group for an alkoxide moiety also allows for the isolation of well-defined salan species, Al(1/2)OiPr (Scheme 2). In solution, the 1H NMR spectrum displays four aromatic resonances and one isopropoxide septet. These complexes were purified by recrystallisation and the corresponding solid state structure was shown to be subtly different to Al(A)OBn/Al(3)Me (Fig. 1); while the trigonal bipyramidal aluminium centre is maintained the wrapping of the ligand around the metal is different. This is illustrated by the respective decrease/increase of the Al–Np/Al–N bonds and an increase/decrease in the Al–O(1)/AlO(2) bonds relative to the parent imine complex. These changes are consistent with the exchange of axial–equatorial position within the trigonal bipyramidal geometry.
The cause of this is thought to be due to a weak interaction between the nitrogen hydrogen and isopropoxide oxygen which, in this conformation, occupy the same plane and while not at the optimum angle for hydrogen bonding are in close proximity (N–H⋯O = 2.227 Å). From structural studies the complexes with ligands 1/2H2 afford different coordination modes than either imine or 3H2 (Scheme 3).
Polymerisations were carried out with lactide purified only by recrystallisation to mimic industrially favoured conditions. It can be immediately seen that the activity of complexes prepared from salans 1/2H2 are markedly improved relative to the corresponding salalen complexes. Solution polymerisations (Table 2) reach reasonable conversion and molecular weight after hours rather than the several days (entry 1 vs. entry 3/4). For the aluminium complex of 1H2, both the methyl and isopropoxide species were trialled with the former having benzyl alcohol added as co-initiator. Encouragingly, very similar results are observed in terms of polymer weight and stereocontrol suggesting that the active alkoxide structure of the two initiators is likely to be identical. As for the imino forms, all initiators prepared in this study demonstrate predictable molecular weights and narrow distributions further highlighting the controlled nature of the polymerisation.
| Entry | Initiator | Time/h | Conv.e/% | P m | M n | PDIg |
|---|---|---|---|---|---|---|
Entries 1/2 from McKeown et al.7 Conditions: toluene, 80 °C.a [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 100 [BnOH] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b [LA] 1.b [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = 100 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.c L-LA.d CH2Cl2, 25 °C.e Determined via1H NMR spectroscopy.f Pm is the probability of isotactic enchainment, determined via homonuclear decoupled 1H NMR spectroscopy with deconvolution and averaging of the five equations.g Molecular weight via GPC analysis (in THF). 1.c L-LA.d CH2Cl2, 25 °C.e Determined via1H NMR spectroscopy.f Pm is the probability of isotactic enchainment, determined via homonuclear decoupled 1H NMR spectroscopy with deconvolution and averaging of the five equations.g Molecular weight via GPC analysis (in THF). |
||||||
| 1a | Al(A)Me | 240 | 88 | 0.63 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.06 |
| 2a | Al(B)Me | 240 | 88 | 0.44 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.08 |
| 3a | Al(1)Me | 3 | 65 | 0.75 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.06 |
| 4b | Al(1)OiPr | 3 | 66 | 0.79 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
1.04 |
| 5b,c | Al(1)OiPr | 3 | 82 | — | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
1.04 |
| 6b,d | Al(1)OiPr | 120 | 60 | 0.83 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.04 |
| 7b | Al(2)OiPr | 0.5 | 76 | 0.59 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.03 |
| 8a | Al(3)Me | 120 | 49 | 0.64 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1.13 |
The initiators based on 1H2 demonstrate an increased isotactic bias (Pm ∼ 0.8) relative to AH2 based initiators with white polymers resulting from the polymerisations. Interestingly, polymerisations in toluene at 80 °C afforded slightly less stereocontrol compared to some of the solvent free reactions (Table 3) at higher temperatures. For the salan complexes based on 2H2, the increase in initiator activity is more pronounced with high conversion being attained within one hour. The tacticity imparted from these complexes is slightly isotactic which again is more pronounced for solvent free polymerisations. The N-methylated salan complex Al(3)Me, was also trialled for the ROP of rac-LA. While the polymerisation appeared well controlled, the activity and stereocontrol of the initiator was found to be comparable to that of the salalen Al(A)Me, with days being required to reach a reasonable conversion of LA. This result indicates it is likely the rearrangement of the ligand around the metal centre that accounts for the increased activity Al(1/2)OiPr which have different coordination motifs to Al(A/3)Me. We believe that this is due to the weak interaction between the NH moiety and the oxygen of the alkoxide.
| Entry | Initiator | Time/h | Conv.h/% | P m | M n | PDIj |
|---|---|---|---|---|---|---|
a Conditions: [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 300 [I] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C.
b [LA] 1, 130 °C.
b [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 900 [I] = 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C.
c [LA] 1, 130 °C.
c [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 900 [I] = 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 150 °C.
d [LA] 1, 150 °C.
d [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 300 [I] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 180 °C.
e [LA] 1, 180 °C.
e [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 900 [I] = 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 180 °C.
f [LA] 1, 180 °C.
f [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 3000 [BnOH] = 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 150 °C.
g [LA] 10, 150 °C.
g [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 3000 [BnOH] = 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 180 °C.
h Determined via1H NMR spectroscopy.
i
P
m is the probability of isotactic enchainment, determined via homonuclear decoupled 1H NMR spectroscopy with deconvolution and averaging of the five equations.
j Molecular weight via GPC analysis (in THF). 10, 180 °C.
h Determined via1H NMR spectroscopy.
i
P
m is the probability of isotactic enchainment, determined via homonuclear decoupled 1H NMR spectroscopy with deconvolution and averaging of the five equations.
j Molecular weight via GPC analysis (in THF).
|
||||||
| 1a | Al(1)OiPr | 0.3 | 75 | 0.81 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1.04 |
| 2b | Al(1)OiPr | 1 | 55 | 0.78 | 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.18 |
| 3c | Al(1)OiPr | 0.5 | 47 | 0.75 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
1.13 |
| 4d | Al(1)OiPr | 0.1 | 79 | 0.73 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.26 |
| 5e | Al(1)OiPr | 0.2 | 45 | 0.70 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.24 |
| 6f | Al(1)OiPr | 6.25 | 53 | 0.70 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1.15 |
| 7g | Al(1)OiPr | 5 | 60 | 0.59 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
1.55 |
| 8a | Al(2)OiPr | 0.1 | 85 | 0.73 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
1.13 |
| 9b | Al(2)OiPr | 0.3 | 50 | 0.73 | 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.09 |
| 10c | Al(2)OiPr | 0.3 | 54 | 0.74 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.17 |
| 11d | Al(2)OiPr | 0.05 | 89 | 0.72 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.25 |
| 12e | Al(2)OiPr | 0.2 | 68 | 0.70 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.29 |
| 13f | Al(2)OiPr | 1 | 70 | 0.70 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.12 |
| 14g | Al(2)OiPr | 1 | 36 | 0.65 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.21 |
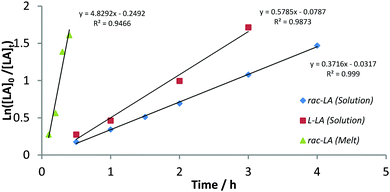
MALDI-ToF analysis of polymers resultant of Al(1/2)OiPr (Table 2, entries 4 and 7) highlighted the controlled nature of the polymerisation in solution showing no evidence of transesterification and yielding molecular weight values consistent with conversion, end groups and GPC analysis. Further investigation into Al(1/2)OiPr revealed controlled polymerisation characteristics with the molecular weight showing linear increase with conversion in solution at 80 °C (see ESI†). First order kinetics was also shown by a linear concentration:time plot for each initiator (Fig. 2 and ESI†). The apparent rate constants, kapp, were found to be 0.37 and 2.7 h−1 for Al(1)OiPr and Al(2)OiPr respectively (Tol, 80 °C, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); evidently, the shorter reaction timescale allows for differentiation between the rates of Al(1/2)OiPr and the increased activity for Al(2)OiPr is attributed to the reduced steric hindrance offered by the dimethyl phenol moiety. The rate constant for the polymerisation of Al(1)OiPr with L-lactide as also evaluated and found to be 0.58 h−1 (Fig. 2 and Table 2, entry 5). NMR spectroscopy showed no evidence of epimerisation for the polymerisation of L-LA. The relative kinetics for the polymerisation of rac- or L-LA allows for the estimation of tacticity; the Pm value from this kinetic perspective is found to be 0.78 which is in accord with that found for the solution polymerisation via NMR spectroscopy. DSC analysis of the polymer prepared at room temperature (Table 2, entry 6) reveals a Tm = 177 °C, further highlighting the isotactic bias of Al(1)OiPr. The mechanistic pathway is unclear due to the use of a racemic ligand. Neither chain end mediated nor enantiomorphic mediated polymerisation can be ruled out and neither can a polymeryl exchange mechanism be discounted; such an exchange has been invoked for Al(III) complexes and could be potentially in operation to form stereoblocks and reduce the overall isotacticity.1g,i,n
1); evidently, the shorter reaction timescale allows for differentiation between the rates of Al(1/2)OiPr and the increased activity for Al(2)OiPr is attributed to the reduced steric hindrance offered by the dimethyl phenol moiety. The rate constant for the polymerisation of Al(1)OiPr with L-lactide as also evaluated and found to be 0.58 h−1 (Fig. 2 and Table 2, entry 5). NMR spectroscopy showed no evidence of epimerisation for the polymerisation of L-LA. The relative kinetics for the polymerisation of rac- or L-LA allows for the estimation of tacticity; the Pm value from this kinetic perspective is found to be 0.78 which is in accord with that found for the solution polymerisation via NMR spectroscopy. DSC analysis of the polymer prepared at room temperature (Table 2, entry 6) reveals a Tm = 177 °C, further highlighting the isotactic bias of Al(1)OiPr. The mechanistic pathway is unclear due to the use of a racemic ligand. Neither chain end mediated nor enantiomorphic mediated polymerisation can be ruled out and neither can a polymeryl exchange mechanism be discounted; such an exchange has been invoked for Al(III) complexes and could be potentially in operation to form stereoblocks and reduce the overall isotacticity.1g,i,n
To assess the applicability of these initiators, further polymerisations of Al(1/2)OiPr were performed. Under standard solvent free conditions (130 °C, 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the activity and control of these initiators is maintained (Table 3, entries 1/8). At this temperature, an increase in monomer-to-initiator ratio (900
1) the activity and control of these initiators is maintained (Table 3, entries 1/8). At this temperature, an increase in monomer-to-initiator ratio (900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is tolerated with minimal reduction of selectivity and molecular weight control and relatively small increase in reaction time. Higher temperature solvent free reactions (150 and 180 °C) were also found to be successful. Generally, higher conversion is achieved more rapidly and control is not significantly reduced. To assess activity under industrial conditions, immortal polymerisations were carried out with reduced metal content and co-initiator to moderate molecular weight ([LA]
1) is tolerated with minimal reduction of selectivity and molecular weight control and relatively small increase in reaction time. Higher temperature solvent free reactions (150 and 180 °C) were also found to be successful. Generally, higher conversion is achieved more rapidly and control is not significantly reduced. To assess activity under industrial conditions, immortal polymerisations were carried out with reduced metal content and co-initiator to moderate molecular weight ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]
[I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 3000
[BnOH] = 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). These conditions are found to be feasible for Al(1/2)OiPr, with a reasonable degree of isoselectivity still being maintained despite extended reaction times at high temperatures (Table 3, entries 6 and 7, 13 and 14). The resultant molecular weight demonstrates these initiators ability to facilitate molecular weight and tacticity control under extremely challenging conditions although it is noted that 5 hours at 180 °C (Table 3, entry 7) does cause a deterioration in molecular weight suggesting a stability limit for Al(1)OiPr.
10). These conditions are found to be feasible for Al(1/2)OiPr, with a reasonable degree of isoselectivity still being maintained despite extended reaction times at high temperatures (Table 3, entries 6 and 7, 13 and 14). The resultant molecular weight demonstrates these initiators ability to facilitate molecular weight and tacticity control under extremely challenging conditions although it is noted that 5 hours at 180 °C (Table 3, entry 7) does cause a deterioration in molecular weight suggesting a stability limit for Al(1)OiPr.
In conclusion we have shown how altering the coordination geometry of simple salans around Al(III) can have dramatic consequences in terms of activity and selectivity. We wish to thank the EPSRC for funding the CDT at Bath (EP/G03768X/1) and Purac for the donation of lactide. For full experimental details see ESI† and http://doi.org/10.15125/BATH-00220 for the data.
Notes and references
- (a) S. Bian, S. Abbina, Z. Lu, E. Kolodka and G. Du, Organometallics, 2014, 33, 2489–2495 CrossRef CAS PubMed; (b) S. L. Hancock, M. F. Mahon and M. D. Jones, Dalton Trans., 2013, 42, 9279–9285 RSC; (c) P. Hormnirun, E. L. Marshall, V. C. Gibson, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2004, 126, 2688–2689 CrossRef CAS PubMed; (d) K. Majerska and A. Duda, J. Am. Chem. Soc., 2004, 126, 1026–1027 CrossRef CAS PubMed; (e) N. Nomura, R. Ishii, M. Akakura and K. Aoi, J. Am. Chem. Soc., 2002, 124, 5938–5939 CrossRef CAS PubMed; (f) N. Nomura, R. Ishii, Y. Yamamoto and T. Kondo, Chem. – Eur. J., 2007, 13, 4433–4451 CrossRef CAS PubMed; (g) T. M. Ovitt and G. W. Coates, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4686–4692 CrossRef CAS; (h) A. Pilone, K. Press, I. Goldberg, M. Kol, M. Mazzeo and M. Lamberti, J. Am. Chem. Soc., 2014, 136, 2940–2943 CrossRef CAS PubMed; (i) K. Press, I. Goldberg and M. Kol, Angew. Chem., Int. Ed., 2015, 54, 14858–14861 CrossRef CAS PubMed; (j) N. Spassky, M. Wisniewski, C. Pluta and A. LeBorgne, Macromol. Chem. Phys., 1996, 197, 2627–2637 CrossRef CAS; (k) Z. Y. Zhong, P. J. Dijkstra and J. Feijen, Angew. Chem., Int. Ed., 2002, 41, 4510–4513 CrossRef CAS; (l) M. D. Jones, L. Brady, P. McKeown, A. Buchard, P. M. Schafer, L. H. Thomas, M. F. Mahon, T. J. Woodman and J. P. Lowe, Chem. Sci., 2015, 6, 5034–5039 RSC; (m) E. L. Whitelaw, G. Loraine, M. F. Mahon and M. D. Jones, Dalton Trans., 2011, 40, 11469–11473 RSC; (n) Z. Zhong, P. J. Dijkstra and J. Feijen, J. Am. Chem. Soc., 2003, 125, 11291–11298 CrossRef CAS PubMed; (o) C. P. Radano, G. L. Baker and M. R. Smith, J. Am. Chem. Soc., 2000, 122, 1552–1553 CrossRef CAS; (p) H.-L. Chen, S. Dutta, P.-Y. Huang and C.-C. Lin, Organometallics, 2012, 31, 2016–2025 CrossRef CAS; (q) L. Chen, W. Li, D. Yuan, Y. Zhang, Q. Shen and Y. Yao, Inorg. Chem., 2015, 54, 4699–4708 CrossRef CAS PubMed; (r) E. D. Cross, L. E. N. Allan, A. Decken and M. P. Shaver, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1137–1146 CrossRef CAS; (s) H. Du, X. Pang, H. Yu, X. Zhuang, X. Chen, D. Cui, X. Wang and X. Jing, Macromolecules, 2007, 40, 1904–1913 CrossRef CAS; (t) C. Kan, J. Ge and H. Ma, Dalton Trans., 2016, 45, 6682–6695 RSC; (u) S. Tabthong, T. Nanok, P. Sumrit, P. Kongsaeree, S. Prabpai, P. Chuawong and P. Hormnirun, Macromolecules, 2015, 48, 6846–6861 CrossRef CAS; (v) H. Du, A. H. Velders, P. J. Dijkstra, J. Sun, Z. Zhong, X. Chen and J. Feijen, Chem. – Eur. J., 2009, 15, 9836–9845 CrossRef CAS PubMed; (w) N. Maudoux, T. Roisnel, V. Dorcet, J.-F. Carpentier and Y. Sarazin, Chem. – Eur. J., 2014, 20, 6131–6147 CrossRef CAS PubMed; (x) P. Hormnirun, E. L. Marshall, V. C. Gibson, R. I. Pugh and A. J. P. White, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15343–15348 CrossRef CAS PubMed; (y) M. Wisniewski, A. LeBorgne and N. Spassky, Macromol. Chem. Phys., 1997, 198, 1227–1238 CrossRef CAS.
- (a) M. D. Jones, S. L. Hancock, P. McKeown, P. M. Schafer, A. Buchard, L. H. Thomas, M. F. Mahon and J. P. Lowe, Chem. Commun., 2014, 50, 15967–15970 RSC; (b) M. Hu, M. Wang, H. Zhu, L. Zhang, H. Zhang and L. Sun, Dalton Trans., 2010, 39, 4440–4446 RSC; (c) J.-C. Buffet and J. Okuda, Chem. Commun., 2011, 47, 4796–4798 RSC; (d) A. J. Chmura, M. G. Davidson, M. D. Jones, M. D. Lunn, M. F. Mahon, A. F. Johnson, P. Khunkamchoo, S. L. Roberts and S. S. F. Wong, Macromolecules, 2006, 39, 7250–7257 CrossRef CAS.
- C. Bakewell, T.-P.-A. Cao, N. Long, X. F. Le Goff, A. Auffrant and C. K. Williams, J. Am. Chem. Soc., 2012, 134, 20577–20580 CrossRef CAS PubMed.
- D. C. Aluthge, B. O. Patrick and P. Mehrkhodavandi, Chem. Commun., 2013, 49, 4295–4297 RSC.
- (a) M. Honrado, A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2014, 33, 1859–1866 CrossRef CAS; (b) H. Wang, Y. Yang and H. Ma, Macromolecules, 2014, 47, 7750–7764 CrossRef CAS; (c) Y. Yang, H. Wang and H. Ma, Inorg. Chem., 2015, 54, 5839–5854 CrossRef CAS PubMed; (d) S. Abbina and G. Du, ACS Macro Lett., 2014, 3, 689–692 CrossRef CAS PubMed.
- C. Bakewell, A. J. P. White, N. J. Long and C. K. Williams, Angew. Chem., Int. Ed., 2014, 53, 9226–9230 CrossRef CAS PubMed.
- P. McKeown, M. G. Davidson, J. P. Lowe, M. F. Mahon, L. H. Thomas, T. J. Woodman and M. D. Jones, Dalton Trans., 2016, 45, 5374–5387 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental data and crystal data. CCDC 1491351–1491354. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc05795k |
| This journal is © The Royal Society of Chemistry 2016 |