Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Neutral versus polycationic coordination cages: a comparison regarding neutral guest inclusion†
György
Szalóki
,
Vincent
Croué
,
Magali
Allain
,
Sébastien
Goeb
* and
Marc
Sallé
*
Laboratoire MOLTECH-Anjou, Université d'Angers, CNRS UMR 6200, 2 bd Lavoisier, 49045 Angers Cedex, France. E-mail: sebastien.goeb@univ-angers.fr; marc.salle@univ-angers.fr
First published on 12th July 2016
Abstract
A neutral self-assembled container synthesized from a concave π-extended tetrathiafulvalene (exTTF) ligand and the cis-Pd(dctfb)2(cod) complex (dctfb = 3,5-dichloro-2,4,6-trifluorobenzene; cod = 1,5-cyclooctadiene) is described. This molecular host exhibits a good binding ability for fused polyaromatic substrates. The corresponding inclusion properties are compared with those of a previously described analogous octacationic cage, offering therefore the opportunity to address the effect of the cavity charge state over the binding of neutral molecules.
The preparation of molecular cages able to encapsulate ionic or neutral organic guests constitutes a major challenge for various applications ranging from selective depollution, reactivity in confined environments, or guest transport. In this context, the coordination-driven self-assembly strategy has been successfully used to build, in a straightforward way, more and more sophisticated metalla-cages,1 including electro-active ones.2 Being most often prepared by the reaction of a polypyridyl ligand (typically di-, tri- or tetra-pyridyl) with a metal cation, they generally correspond to polycationic cages.1,3 On this basis, we showed recently that the cationic coordination-cage M4L28+ (Scheme 1 and Fig. 2b) is able to bind the B12F122− guest in a reversible way through a redox-driven assembly/disassembly process.4 Host–guest interactions are by nature more challenging to address in the case of neutral guests, which renders the design of appropriate receptors more delicate. On these grounds, an efficient redox control of the binding mode5,6 has been recently demonstrated from a tetracationic organic covalent receptor. When considering neutral guests, the polycationic character of the cages usually obtained through coordination-driven self-assembly may hamper both the kinetics and the thermodynamics of the binding, in particular because of the presence of highly competitive counter anions which obstruct the cavity.1m,7 On this basis, the design of neutral receptors through the self-assembled metal-driven strategy appears promising. Examples of neutral discrete metalla-assemblies are known and are synthetized either from the reaction of an anionic donating ligand (typically bearing carboxylate groups) and a cationic metal center,8 or from the reaction between a neutral ligand and a neutral metallic precursor.1m,7 Some data related to the binding ability of such a neutral coordination cage for neutral guests have already been provided.7 Nevertheless, to the best of our knowledge, no comparative experimental study is available which addresses in a quantitative manner the binding affinity of a neutral guest for a charged coordination cage versus a neutral one, whereas this issue has already been reported in the case of an ionic guest.9 Such a comparative study, which is of importance for a better understanding of intermolecular forces which govern the recognition phenomena, needs to define a proper cavity whose geometry and size are the same for both the neutral and cationic states.
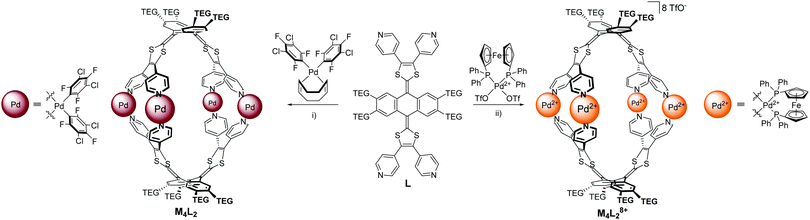 | ||
| Scheme 1 Synthesis of cage M4L2 and M4L28+. (i) Acetone, rt, 48 h, 87%; (ii) nitromethane, 40 °C, 5 min, 83%. | ||
We describe herein a metal-directed neutral self-assembled cage M4L2 constructed from a tetra-pyridyl exTTF ligand and a neutral cis-oriented square planar bis-aryl palladium complex (cis-Pd(dctfb)2(cod)).10 The resulting assembly offers a unique opportunity to quantitatively compare the binding affinities of planar polyaromatic guests for two similar cavities (M4L2 and M4L28+) (Scheme 1) which essentially differ in the charge state of the metal center and therefore in the presence, or not, of counter anions.11
The tetrapyridyl-exTTF ligand L (Scheme 1) was synthesized in three steps from 2,3,6,7-tetrahydroxyanthracene-9,10-dione, in a 57% overall yield as previously described.4 The presence of the four peripheral triethylene glycol (TEG) chains on the anthracene core ensures the solubility of the resulting cages in various solvents. The self-assembly reaction of ligand L and complex cis-Pd(dctfb)2(cod) was carried out for 48 h in acetone at room temperature. The reaction converged into a single discrete compound M4L2 (Scheme 1) that could be isolated in 87% yield via simple filtration.
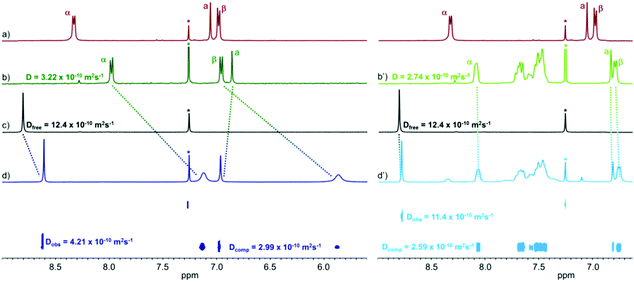
The complex M4L2 presents a high field shift of the α and β pyridyl signals in the 1H NMR spectrum compared to ligand L (Fig. 1a, b and Fig. S1, ESI†), as expected from the coordination to the metal center. 1H DOSY NMR exhibits only one alignment of signals with an extracted D value of 3.22 × 10−10 m2 s−1 in CD3NO2/CDCl3 (1/1) and 3.35 × 10−10 m2 s−1 in CDCl3 (Fig. S3 and S4, ESI†) and confirms the formation of only one discrete species. The corresponding hydrodynamic radius12 estimated from the Stokes–Einstein equation13 (T = 298 K) is of 11.4 Å, a value which is in accordance with the formation of a M4L2 species. ESI-FTICR mass spectrometry experiments carried out with M4L2 are silent due to the lack of charges. Taking advantage of the well-known ability of TEG chains to bind alkaline cations, an excess of KOTf (8 equivalents) was added to a solution of M4L2 (C = 2 × 10−3 M, CH2Cl2/CH3NO2 5/5) before ionization. Thus, the generated ionic species could be studied and confirmed the stoichiometry of the complex, as illustrated by characteristic multi-charged peaks [M4L2·(KOTf)4-4OTf]4+ (m/z = 1213.94) and [M4L2·(KOTf)4-3OTf]3+ (m/z = 1668.58) as well as by the good accordance between the experimental and the theoretical isotopic patterns (Fig. S5, ESI†).
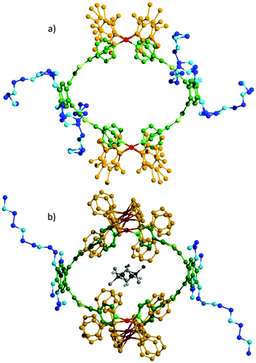
Single crystals of M4L2 could be grown by slow diffusion of acetonitrile in a chloroform solution and an XRD experiment confirmed the formation of a M4L2 cage (Fig. 2a and Fig. S30, ESI†) which exhibits an internal ovoid cavity of ca. 15 Å length over 13 Å width, close to that of M4L28+ (ca. 15.5 Å × 11.5 Å) (Fig. 2b).14 This shape similitude offers a unique opportunity to compare the ability of these respectively neutral and charged metalla-cages for encapsulating a neutral guest, since they essentially differ by (i) their charge state on the metal and by (ii) the presence (M4L28+) or absence (M4L2) of counter anions. In a previous work, we demonstrated that a polycationic cage, similar to M4L28+ but devoid of peripheral TEG chains, allows the inclusion of perylene according to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in nitromethane.15
1 stoichiometry in nitromethane.15
We therefore studied the inclusion of different neutral fused polyaromatic guests into the ovoid cavities of M4L2 and M4L28+ by 1H NMR and 1H DOSY NMR. A solvent mixture (CD3NO2/CDCl3 1/1) has to be used in order to ensure the solubility of all the species and to allow a comparative study. As an illustrative example of the respective behavior of the neutral and the charged cages upon encapsulation, the 1H NMR binding study of coronene is shown in Fig. 1. Importantly, whereas only a slight difference is observed for M4L28+ upon addition of one equivalent of the guest (Fig. 1b′ and d′), the 1H NMR signals of metalla-cage M4L2 and of coronene are significantly high-field shifted (Fig. 1b–d). The binding is accompanied in the case of M4L2 by a significant decrease of the coronene diffusion coefficient D value (D = 4.21 × 10−10 m2 s−1vs. Dfree = 12.40 × 10−10 m2 s−1) in the 1H DOSY NMR spectrum (Fig. 1c and d). In contrast, the coronene D value remains almost unchanged in the presence of M4L28+ (D = 11.40 × 10−10 m2 s−1vs. Dfree = 12.40 × 10−10 m2 s−1). As already demonstrated for the charged cage, the formation of a 1/1 host–guest complex was also found via a Job Plot analysis for M4L2 (Fig. S32, ESI†). From these 1H DOSY NMR data, Ka binding constants of 2.6 × 104 (M4L2) and 63 (M4L28+) could be calculated (see ESI† for details), meaning a ratio of ca. 400 in favor of the neutral cage. The complexation of other planar aromatic guests was studied and their respective binding constants are compiled in Table 1. As it can be noted from these values, the binding abilities increase with the guest size and, remarkably, the neutral M4L2 cage systematically presents a higher affinity (one to several orders of magnitude higher) for the tested neutral polyaromatic guests. These results clearly demonstrate the positive effect of the absence of charges and of anions on the periphery of the ovoid cavity, for the encapsulation of neutral guests.16 Increasing the proportion of nitromethane (a poor solvent for the guest under consideration) in the solvent mixture results in a significant increase of the binding constant (Ka = 1.1 × 105 with M4L2 and coronene). Single crystals of the coronene⊂M4L2 inclusion complex could be grown at room temperature from the latter solvent mixture. The XRD experiment confirms the encapsulation of one coronene unit located in the middle of the cavity defined by the exTTF concave fragments containing both 1,3-dithiol rings (Fig. 3). It is worth noting that in order to maximize the host–guest interactions, the Pd–Pd distances are significantly reduced upon complexation related to the free cage M4L2, as can be seen from the cavity size which changes from 15 Å × 13 Å (Fig. 2a) to 16 Å × 10.5 Å in coronene⊂M4L2,14 illustrating the unexpectedly flexible character of the cavity. Binding studies of coronene with the M4L28+ assembly subjected to a TfO− anion exchange (i.e. BF4− and B12F122−) were carried out and monitored by 1H DOSY NMR in CD3NO2/CDCl3 8/2. Ka values of 200 and 58 were found for BF4− and B12F122− respectively (170 for TfO−) (Fig. S12, S27 and S28, ESI†) showing that the nature of the counter anion does influence the binding of the coronene guest and justify the superiority of the neutral host over the polycationic one. Finally, an inclusion control study was led by 19F DOSY NMR experiments with the B12F122− anion (Fig. S29–S31, ESI†). Ka values of 25 and >106 were obtained for M4L2 and M4L28+ respectively, confirming that the polycationic cage binds much more strongly anionic guests than the neutral one does.
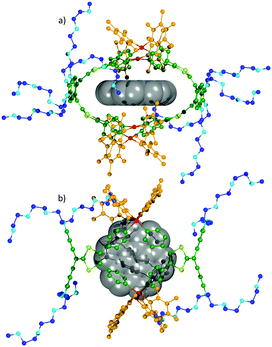 | ||
| Fig. 3 X-Ray crystal structure of coronene⊂M4L2 (a) side view, (b) top view. Colors used: exTTF skeleton (green), PEG chains (blue), Pd complex (orange), coronene (grey). | ||
In summary, an electron-rich exTTF-based M4L2 neutral cage which exhibits a good ability to bind planar polyaromatic guests in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was synthesized. This neutral assembly could be compared to the previously described analogous M4L28+ system, which essentially differs in the charges on the metal and therefore by the presence of counter anions surrounding the cavity. This comparative experimental study affords first quantitative evidence of the superiority of neutral hosts for the binding of neutral guests (with Ka values which are one to several orders of magnitude higher in this study), an observation which is of importance for the rational design of strong host/guest systems in future. On these grounds, the design of new neutral metalla-cages appears highly relevant.7 In particular, the present work illustrates the promising perspectives offered by the square planar cis-Pd(dctfb)2(cod) complex10 for preparing neutral cages upon interaction with various poly-pyridyl ligands, in a straightforward way and under mild conditions. Therefore, the scope of this approach can in principle be extended to most of the abundant poly-pyridyl ligands available in the literature and previously used to build polycationic cages.
1 stoichiometry was synthesized. This neutral assembly could be compared to the previously described analogous M4L28+ system, which essentially differs in the charges on the metal and therefore by the presence of counter anions surrounding the cavity. This comparative experimental study affords first quantitative evidence of the superiority of neutral hosts for the binding of neutral guests (with Ka values which are one to several orders of magnitude higher in this study), an observation which is of importance for the rational design of strong host/guest systems in future. On these grounds, the design of new neutral metalla-cages appears highly relevant.7 In particular, the present work illustrates the promising perspectives offered by the square planar cis-Pd(dctfb)2(cod) complex10 for preparing neutral cages upon interaction with various poly-pyridyl ligands, in a straightforward way and under mild conditions. Therefore, the scope of this approach can in principle be extended to most of the abundant poly-pyridyl ligands available in the literature and previously used to build polycationic cages.
This work has been supported by the ANR JCJC program (ANR-14-CE08-0001 BOMBER). The authors gratefully acknowledge the MENRT for a PhD grant (VC). They also acknowledge Dr I. Freuze and B. Siegler (PIAM, Univ. Angers), as well as Drs F. Aubriet and V. Carré (CNRS-Univ. Lorraine (TGE “FT-ICR”)) for their assistance in spectroscopic analyses and finally Drs P. Fertey and S. Ravy (Synchrotron Soleil – CRISTAL beamline – project 20130173).
Notes and references
- (a) For recent reviews see: N. Ahmad, H. A. Younus, A. H. Chughtai and F. Verpoort, Chem. Soc. Rev., 2015, 44, 9 RSC; (b) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed; (c) S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419 RSC; (d) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848 RSC; (e) S. Mukherjee and P. S. Mukherjee, Chem. Commun., 2014, 50, 2239 RSC; (f) T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang and K.-W. Chi, Acc. Chem. Res., 2013, 46, 2464 CrossRef CAS PubMed; (g) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703 RSC; (h) A. Mishra, S. C. Kang and K.-W. Chi, Eur. J. Inorg. Chem., 2013, 5222 CrossRef CAS; (i) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728 RSC; (j) M. D. Ward and P. R. Raithby, Chem. Soc. Rev., 2013, 42, 1619 RSC; (k) H. Amouri, C. Desmarets and J. Moussa, Chem. Rev., 2012, 112, 2015 CrossRef CAS PubMed; (l) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2012, 113, 734 CrossRef PubMed; (m) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed; (n) L. R. MacGillivray, Angew. Chem., Int. Ed., 2012, 51, 1110 CrossRef CAS PubMed; (o) Y. Inokuma, M. Kawano and M. Fujita, Nat. Chem., 2011, 3, 349 CrossRef CAS PubMed; (p) S. De, K. Mahata and M. Schmittel, Chem. Soc. Rev., 2010, 39, 1555 RSC; (q) P. Jin, S. J. Dalgarno and J. L. Atwood, Coord. Chem. Rev., 2010, 254, 1760 CrossRef CAS; (r) Y.-F. Han, W.-G. Jia, W.-B. Yu and G.-X. Jin, Chem. Soc. Rev., 2009, 38, 3419 RSC; (s) B. H. Northrop, Y.-R. Zheng, K.-W. Chi and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554 CrossRef CAS PubMed; (t) P. J. Stang, J. Org. Chem., 2009, 74, 2 CrossRef CAS PubMed; (u) B. Therrien, Eur. J. Inorg. Chem., 2009, 2445 CrossRef CAS; (v) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418 CrossRef CAS PubMed; (w) M. W. Cooke, D. Chartrand and G. S. Hanan, Coord. Chem. Rev., 2008, 252, 903 CrossRef CAS; (x) S. J. Dalgarno, N. P. Power and J. L. Atwood, Coord. Chem. Rev., 2008, 252, 825 CrossRef CAS; (y) B. H. Northrop, D. Chercka and P. J. Stang, Tetrahedron, 2008, 64, 11495 CrossRef CAS PubMed.
- V. Croué, S. Goeb and M. Sallé, Chem. Commun., 2015, 51, 7275 RSC.
- For anionic metalla-cages, see also: (a) D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 CrossRef CAS; (b) Z. J. Wang, C. J. Brown, R. G. Bergman, K. N. Raymond and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 7358 CrossRef CAS PubMed.
- V. Croué, S. Goeb, G. Szalóki, M. Allain and M. Sallé, Angew. Chem., Int. Ed., 2016, 55, 1746 CrossRef PubMed.
- M. Frasconi, I. R. Fernando, Y. Wu, Z. Liu, W.-G. Liu, S. M. Dyar, G. Barin, M. R. Wasielewski, W. A. Goddard and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 11057 CrossRef CAS PubMed.
- For a redox-based switching of guest binding performed upon stimulating the guest, see e.g. W.-Y. Sun, T. Kusukawa and M. Fujita, J. Am. Chem. Soc., 2002, 124, 11570 CrossRef CAS PubMed.
- P. Thanasekaran, C.-H. Lee and K.-L. Lu, Coord. Chem. Rev., 2014, 280, 96 CrossRef CAS.
- N. Ahmad, A. H. Chughtai, H. A. Younus and F. Verpoort, Coord. Chem. Rev., 2014, 280, 1 CrossRef CAS.
- J. Kuwabara, C. L. Stern and C. A. Mirkin, J. Am. Chem. Soc., 2007, 129, 10074 CrossRef CAS PubMed.
- L. J. Marshall and J. de Mendoza, Org. Lett., 2013, 15, 1548 CrossRef CAS PubMed.
- Note in addition that, whereas M4L2 involves two dctfb (3,5-dichloro-2,4,6-trifluorobenzene) as Pd co-ligands, M4L28+ involves dppf.
- Calculated from the experiment led in a pure solvent (CDCl3) (Fig. S3, ESI†), for which the viscosity of the medium is known.
- (a) Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520 CrossRef CAS PubMed; (b) L. Avram and Y. Cohen, Chem. Soc. Rev., 2015, 44, 586 RSC.
- The cavity size values are obtained by measuring the distance between (i) the opposite central anthracene rings on one hand and (ii) two opposite palladium atoms on the other hand.
- S. Bivaud, S. Goeb, V. Croué, P. I. Dron, M. Allain and M. Sallé, J. Am. Chem. Soc., 2013, 135, 10018 CrossRef CAS PubMed.
- Performing the binding studies at a similar ionic strength for M4L28+ and M4L2, by addition of 8 equivalents of tetrabutylammonium trifluoromethane-sulfonate in the latter case, does not change the result.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis protocols, experimental methods, X-ray diffraction studies and additional spectroscopic data for ligand L and complexes M4L2 and M4L28+. CCDC 1441971 and 1441826. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04610j |
| This journal is © The Royal Society of Chemistry 2016 |