Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[Ni(OH)3W6O18(OCH2)3CCH2OH]4−: the first tris-functionalized Anderson-type heteropolytungstate†
Nadiia I.
Gumerova‡
a,
Alexander
Roller
b and
Annette
Rompel
 *a
*a
aUniversität Wien, Fakultät für Chemie, Institut für Biophysikalische Chemie, Althanstr. 14, 1090 Wien, Austria. E-mail: annette.rompel@univie.ac.at; Web: http://www.bpc.univie.ac.at
bUniversität Wien, Fakultät für Chemie, Institut für Anorganische Chemie, Währinger Str. 42, 1090 Wien, Austria
First published on 15th June 2016
Abstract
Na2[TMA]2[Ni(OH)3W6O18(OCH2)3CCH2OH]·9H2O represents the first covalent tris-functionalized Anderson-type heteropolytungstate and was characterized by single-crystal X-ray diffraction, electrospray ionization mass spectrometry, TGA and IR spectroscopy. Zeta potential measurements in solutions containing human serum albumin were performed to investigate electrostatic interactions with [Ni(OH)3W6O18(OCH2)3CCH2OH]4−.
Polyoxometalates (POMs) are an exceptional brand of coordination compounds consisting of early transition metal atoms linked by shared oxygen atoms.1 Due to their wide range of size, structure and composition, they possess unique thermal, redox, magnetic, optical and bioactive properties and exhibit an enormous potential for application in various fields.2 Especially Lindqvist- and Keggin-type POMs have been shown to exhibit biological activity in vitro as well as in vivo ranging from anti-cancer, antibiotic, and antiviral to antidiabetic effects.3 The design of organically functionalized (inorganic) POM hybrids via a controllable synthesis has gained much attention since the combination of organic and inorganic components into one single compound provides new properties that benefit from the strengths of both units for multi-functional hybrid materials.4
From the first by Knoth5 reported formation of O-alkylated anions, [PM12O39(OMe)]2− (M = Mo, W), the direct covalent functionalization with tris-alkoxo organic ligands (tris(hydroxymethyl)methane (RC(CH2OH)3)) has been widely applied for different POM archetypes.6 The controllable synthesis of functionalized Anderson-type polyoxomolybdates (POMo) has gained much attention7 since the first Anderson-type hybrid structure was described by Hasenknopf in 2002.7a This functionalization is achieved by replacing three or six protons of the B-type Anderson-structure, which are attached to the μ3-O or even to the less basic μ2-O atoms, with organic tris-ligands. Single- or double-side grafted δ-, χ- or ψ-isomers of functionalized Anderson-type POMos, [M(OH)6Mo6O18]n− (M = Zn2+, Ni2+, Cr3+, Mn3+, Al3+, Fe3+, Ga3+), can be obtained via the re-arrangement of the octamolybdate-anion or by applying a pre-synthesized Anderson polyanion under different reaction conditions.7,8 However, to the best of our knowledge nobody succeeded so far in obtaining organically functionalized Anderson-type polyoxotungstates (POTs). The derivatization of POTs applying tripodal ligands is only known for mixed anions exhibiting two addenda atoms.6 It should be noted that in these structures the anchoring of organic groups is only observed onto the V3O13 fragments of the Dawson or Keggin anions or onto the Ni6-core in Ni6PW9.9 The disinclination to easily form W–O–C bonds can be explained by the inertness of the bridging oxygen atoms in the WO6 octahedra. The covalent functionalization of lacunary POTs is accomplished by the formation of W–O–X bonds (X = N, Si, P, As, Sn) with the more nucleophilic terminal O atoms at the surface of the lacuna.10
The application of [TeW6O24]6− (TEW, A-type Anderson-POT) has recently been expanded to its successful use as an additive in protein crystallization.11 Here, [TeW6O24]6− demonstrated superiority over other POM archetypes owing to its good water solubility, pH-stability, disk-shape structure and relatively high negative charge. In the presence of proteins [TeW6O24]6− exhibits no proteolytic activity,11d does not alter the protein structure but provides useful anomalous signal for phasing due to the high molar weight of tungsten. So far and uniquely observed, [TeW6O24]6− mediates crystal contacts11b–e between protein molecule favoring crystal growth. Some protein crystals that were co-crystallized with [TeW6O24]6− possess a higher resolution than the crystals obtained without [TeW6O24]6−.11e There is an urgent need for covalently functionalized Anderson-type POTs to enable specific binding options via organic functional groups between proteins and the Anderson-type POT. Therefore, we developed a method to prepare a tris-functionalized Anderson POT. Herein, we report the first synthesis of a tris-functionalized Anderson-type polyoxotungstate-anion, [Ni(OH)3W6O18(OCH2)3CCH2OH]4− (NiW6-Tris-CH2OH), and its characterization by single-crystal X-ray analysis, electrospray ionization mass spectrometry (ESI-MS), IR as well as TGA. Zeta potential measurements were performed to investigate and compare the electrostatic interactions of NiW6-Tris-CH2OH, with its pure inorganic counterparts [Ni(OH)6W6O18]4−, [Cr(OH)3W6O21]6− and [TeW6O24]6− on human serum albumin HSA.
The full protonation of the six μ3-O atoms (forming the coordination sphere of the heteroatom) is only present in B-type Anderson anions1 and so far exclusively observed for [Ni(OH)6W6O18]4−.12 Due to this fact, [Ni(OH)6W6O18]4− has been chosen as the starting compound for the tris-functionalization. When choosing between different tris(hydroxymethyl)methane ligands, we took into consideration a previous report7b showing the preference of Ni2+ for the amino functionality of the Tris-NH2 by forming Ni–NH2 complexes and no Tris-NH2-functionalized Ni-centered Anderson POMo product. Therefore, no amino-containing tris ligand was used, but pentaerythritol CH2OH–C(CH2OH)3 (Tris-CH2OH) was chosen as the ligand for grafting onto the Ni-containing POT [Ni(OH)6W6O18]4−. By applying Tris-CH2OH one OH-group still stays available after anchoring and can be used for further post-functionalization by esterification with acid anhydrides13 making Tris-CH2OH the ligand of choice.
NiW6-Tris-CH2OH is assembled by a condensation reaction of Tris-CH2–OH with the [Ni(OH)6W6O18]4− anion in an acidified aqueous solution (Scheme 1). Initially, an aqueous solution of Ni2+–WO42−–H+ with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (Ni
6 (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H+) at pH 6.5 was prepared. The reaction mixture was kept 10 days at ambient conditions in order to increase the concentration of [Ni(OH)6W6O18]4−. The pH of the obtained blue solution was reduced to 3.5 with diluted nitric acid. Tris-CH2OH was added in fivefold excess before refluxing the solution for 5 h followed by the addition of tetramethylammonium (TMA) chloride as counter ions. Providing less excess of the tripodal ligand leads to no functionalization, however, a higher excess of Tris-CH2OH did not lead to the double sided functionalized anion and solely the one-side functionalized anion was obtained.
H+) at pH 6.5 was prepared. The reaction mixture was kept 10 days at ambient conditions in order to increase the concentration of [Ni(OH)6W6O18]4−. The pH of the obtained blue solution was reduced to 3.5 with diluted nitric acid. Tris-CH2OH was added in fivefold excess before refluxing the solution for 5 h followed by the addition of tetramethylammonium (TMA) chloride as counter ions. Providing less excess of the tripodal ligand leads to no functionalization, however, a higher excess of Tris-CH2OH did not lead to the double sided functionalized anion and solely the one-side functionalized anion was obtained.
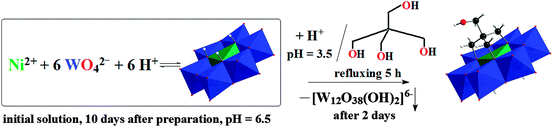 | ||
| Scheme 1 The synthesis of NiW6-Tris-CH2OH; color code: WO6, blue octahedra; NiO6, green octahedron; O, red; C, black; H, white. | ||
Some competing processes can take place in the reaction mixture: (1) self-assembly of NiW6-Tris-CH2OH from monomeric anions, (2) formation of NiW6-Tris-CH2OH by grafting tripodal ligand onto formed [Ni(OH)6W6O18]4− and (3) partial formation of isopolyparticles as byproducts due to the influence of the low pH. After filtration of the reaction solution a salt has been isolated and identified by single-crystal X-ray diffraction measurements and IR spectroscopy as metatungstate TMA6[W12O38(OH)2] (see Fig. S2, ESI†). From the blue mother liquor the single-side tris-functionalized Anderson-type δ isomer [Ni(OH)3W6O18(OCH2)3CCH2OH]4− (see Fig. 1) has been obtained in a quite good yield of 55%. The main advantage of this synthesis route is the possibility to straightforwardly obtain the functionalized Anderson-type cluster in a one-pot reaction which paves the way for more Ni-Anderson tungstate hybrids by applying other tripodal ligands or exploiting the reactivity of the free OH-group in Tris-CH2OH.
 | ||
| Fig. 1 ORTEP drawing of NiW6-Tris-CH2OH (50% probability ellipsoid, H atoms represented in ball mode): W, blue; Ni, green; O, red; C, black; H, light grey. | ||
Our attempts to carry out the synthesis without addition of extra acid to the initial solution of Ni2+–WO42−–H+ or to an aqueous solution of Na4[Ni(OH)6W6O18] (pH = 6.5) that had been refluxed 3, 5 or 12 h applying various amounts of Tris-CH2OH were not successful. In all cases the crystallization of a non tris-decorated product was observed.
The impact of the pH during the Tris-POMo synthesis of [Cr(OH)6Mo6O18]4− and [Mn(OH)6Mo6O18]4−, leading to differently formed isomers has been very recently investigated and discussed by the Wei group.7g,h They showed that the addition of protons to the reaction mixture during the functionalization leads to the protonation of one of the μ2-O atoms in the Anderson anion. This protonated μ2-O represents a highly reactive site where the tris-ligand is anchored on two μ3-O atoms and one μ2-O atom forming the χ isomer. However, in the case of [Ni(OH)6W6O18]4−, the presence of extra protons does not lead to the formation of the χ isomer but to the δ isomer, NiW6-Tris-CH2OH, which is most likely caused by the inertness of the μ2-O atoms inhibiting the formation of a W–O–C bond with alkoxo ligands from the W–O–W fragment.
Na2[TMA]2[Ni(OH)3W6O18(OCH2)3CCH2OH]·9H2O crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The structure is composed of a [Ni(OH)3W6O18(OCH2)3CCH2OH]4− anion, two tetramethyl-ammonium and two sodium cations. NiW6-Tris-CH2OH shows the common Anderson heteropolyanion structure which consists of six WO6 octahedra arranged hexagonally around the central {NiO6} octahedron (Fig. 1). The ligand replaces three protons from the Ni(OH)6 core in order to attach to the disk shape anion. The Ni–O bond lengths are in the range from 2.018(11) to 2.042(15) Å and the μ3-O–C bond lengths are in the range from 1.373(2) to 1.389(2) Å, which is in good agreement with the corresponding bond lengths of the Anderson-anion [H2NiMo6O18{(OCH2)3CCH2OH}2]2−.7a The structure analysis indicates that the lengths of all three types of W–O bonds (W–μ2-O, W–μ3-O, W
. The structure is composed of a [Ni(OH)3W6O18(OCH2)3CCH2OH]4− anion, two tetramethyl-ammonium and two sodium cations. NiW6-Tris-CH2OH shows the common Anderson heteropolyanion structure which consists of six WO6 octahedra arranged hexagonally around the central {NiO6} octahedron (Fig. 1). The ligand replaces three protons from the Ni(OH)6 core in order to attach to the disk shape anion. The Ni–O bond lengths are in the range from 2.018(11) to 2.042(15) Å and the μ3-O–C bond lengths are in the range from 1.373(2) to 1.389(2) Å, which is in good agreement with the corresponding bond lengths of the Anderson-anion [H2NiMo6O18{(OCH2)3CCH2OH}2]2−.7a The structure analysis indicates that the lengths of all three types of W–O bonds (W–μ2-O, W–μ3-O, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oterminal) are also close to the one of the parent species [Ni(OH)6W6O18]4− and to the corresponding POMo analogues (Table 1).
Oterminal) are also close to the one of the parent species [Ni(OH)6W6O18]4− and to the corresponding POMo analogues (Table 1).
[Ni(OH)6W6O18]4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
[Ni(OH)3W6O18(OCH2)3CCH2OH]4− | [Ni(OH)6Mo6O18]4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
[H2NiMo6O18{(OCH2)3CCH2OH}2]2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7a 7a |
|
|---|---|---|---|---|
| Distances, Å: | ||||
| M–μ2-O | 2.229(11)–2.243(12) | 2.227(10)–2.258(12) | 2.223(9)–2.238(9) | 2.078(8)–2.317(10) |
| M–μ3-O | 1.915(10)–1.958(11) | 1.912(10)–1.946(11) | 1.911(12)–1.972(13) | 1.861(13)–1.964(12) |
| M–Oterminal | 1.723(13)–1.744(11) | 1.736(11)–1.742(10) | 1.708(11)–1.726(12) | 1.695(12)–1.708(10) |
| Ni–μ3-O | 2.027(4)–2.051(3) | 2.018(4)–2.042(3) | 2.024(8)–2.050(7) | 2.010(2)–2.085(2) |
| μ3(μ2)-O–C | — | 1.373(2)–1.389(2) | — | 1.430(3)–1.453(2) |
The NiW6-Tris-CH2OH anion interacts with sodium counter cations via their W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O terminal oxygen atoms (O7, O11), hence the coordination sphere of Na+ consists of two oxygen atoms from two neighboring polyanions and three water molecules exhibiting trigonale bipyramide geometry (see Fig. S1, ESI†). That way, alternate heteropolyanions and sodium NaO5 polyhedra form parallel chains interacting through intermolecular hydrogen bonds. Crystal lattice water molecules, the terminal oxygen atoms in the anion, the OH functionality in the grafted organic ligands and the three protons found on the undecorated side form a three dimensional system of H-bonds in the crystal structure (see Fig. S1, ESI†).
O terminal oxygen atoms (O7, O11), hence the coordination sphere of Na+ consists of two oxygen atoms from two neighboring polyanions and three water molecules exhibiting trigonale bipyramide geometry (see Fig. S1, ESI†). That way, alternate heteropolyanions and sodium NaO5 polyhedra form parallel chains interacting through intermolecular hydrogen bonds. Crystal lattice water molecules, the terminal oxygen atoms in the anion, the OH functionality in the grafted organic ligands and the three protons found on the undecorated side form a three dimensional system of H-bonds in the crystal structure (see Fig. S1, ESI†).
IR spectroscopy was applied to investigate the anion NiW6-Tris-CH2OH (see Fig. S3, ESI†). The characteristic peaks of the core structure are all in agreement with the peaks observed in the spectrum of Na4[Ni(OH)6W6O18]·16H2O.12 The stretching vibrations of the terminal W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units appear at 955 and 940 cm−1. The peaks at 884 cm−1 and in the region of 470–750 cm−1 correspond to the antisymmetric and symmetric deformation vibrations of W–O–W and W–O–Ni bridging fragments. The re-duction of symmetry from D3d in [Ni(OH)6W6O18]4− to C2h in NiW6-Tris-CH2OH leads to the reduction in the intensity of the W–O–W vibrations bands at 560 and 725 cm−1. The three peaks appearing at 1105, 1072 and 1035 cm−1 could be assigned to C–O stretching vibrations, indicating the successful grafting of Tris-CH2OH.
O units appear at 955 and 940 cm−1. The peaks at 884 cm−1 and in the region of 470–750 cm−1 correspond to the antisymmetric and symmetric deformation vibrations of W–O–W and W–O–Ni bridging fragments. The re-duction of symmetry from D3d in [Ni(OH)6W6O18]4− to C2h in NiW6-Tris-CH2OH leads to the reduction in the intensity of the W–O–W vibrations bands at 560 and 725 cm−1. The three peaks appearing at 1105, 1072 and 1035 cm−1 could be assigned to C–O stretching vibrations, indicating the successful grafting of Tris-CH2OH.
Evidence for the existence of an intact NiW6-Tris-CH2OH polyanion in solution and its exact stoichiometric composition was obtained from ESI-MS. The ESI-MS spectrum of NiW6-Tris-CH2OH demonstrates its complex character and exhibits a peaks envelope at m/z = 828.8 and m/z = 839.9 which can be unambiguously assigned to the double charged anions Na2−xHx[Ni(OH)3W6O18(OCH2)3CCH2OH]2− (x = 0, 1; calculated mass of 828.8 and 839.9) indicating the presence of the intact one-side grafted cluster in the solution. A series of peak envelopes in the rest of the spectra could be assigned to different common POTs fragments (HWO4−, W2O72−, HW2O7−, NaW2O7−, W3O102−, HW3O102−etc.) and some specific decomposition products, e.g. NaNiW4O13(OH)3−, Na2H3[Ni(OH)3W3O10(OCH2)3CCH2OH]−, NaH[Ni(OH)3W5O14(OCH2)3CCH2OH]−etc. (see Fig. S4 and S5, ESI†).
The TGA data were used to examine the weight loss and thermal stabilities of the synthesized hybrid POT. The TG curve shows three weight-loss regions up to 600 °C due to dehydration followed by disintegration of the various organic complexes (see Fig. S6, ESI†). The dehydration takes place in two stages, at 33–160 °C and 160–230 °C, indicating the presence of differently strong coordinated water in the structure. The loss of three OH-groups from the coordination sphere of the of Ni2+ could not be found as a separate step on the TG curve and presumably takes place together with the loss of the organic ligand between 230 and 530 °C.
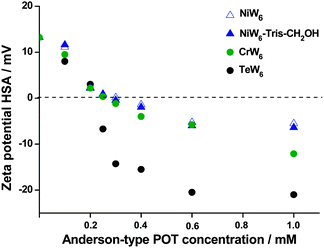
To investigate the interactions between the negatively charged Anderson-tungstate anions and the positively charged surface regions in human serum albumin (HSA), zeta potential measurements on a series of POTs with Ni, Cr and Te as heteroatom ([Ni(OH)6W6O18]4−, [Cr(OH)3W6O21]6−, [TeW6O24]6−) and the tris-functionalized product NiW6-Tris-CH2OH were applied. The zeta potential was measured in NaOAc buffered (50 mM, pH 4.0) solutions containing 1 mg mL−1 (0.015 mM) of HSA. The concentration of heteropolyanions {XW6} was varied from 0 to 1 mM and all solutions were incubated overnight at 4 °C prior to the measurements. The molar ratio between protein and {XW6} anions varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 1
0 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67. The results are illustrated in Fig. 2 and indicate a progressive decrease of the zeta potential of the surface of HSA with increasing POT concentration for all compounds. The charge inversions took place at the following concentrations: 0.25 mM for [TeW6O24]6−, 0.3 mM for [Cr(OH)3W6O21]6−, 0.3 mM for NiW6-Tris-CH2OH and 0.35 mM for [Ni(OH)6W6O18]4−. The slightly lower concentration of NiW6-Tris-CH2OH for charge inversion in comparison to that of [Ni(OH)6W6O18]4− may be caused by additional interaction between the OH functionality and the protein. The interactions of triply charged Anderson-type POMos with bovine serum albumin also showed charge inversions of BSA at low concentrations of anions decorated with Tris-CH2OH bearing a high negative surface charge.7f
67. The results are illustrated in Fig. 2 and indicate a progressive decrease of the zeta potential of the surface of HSA with increasing POT concentration for all compounds. The charge inversions took place at the following concentrations: 0.25 mM for [TeW6O24]6−, 0.3 mM for [Cr(OH)3W6O21]6−, 0.3 mM for NiW6-Tris-CH2OH and 0.35 mM for [Ni(OH)6W6O18]4−. The slightly lower concentration of NiW6-Tris-CH2OH for charge inversion in comparison to that of [Ni(OH)6W6O18]4− may be caused by additional interaction between the OH functionality and the protein. The interactions of triply charged Anderson-type POMos with bovine serum albumin also showed charge inversions of BSA at low concentrations of anions decorated with Tris-CH2OH bearing a high negative surface charge.7f
In summary, 14 years after the first successful tris functionalization of an Anderson type POMo, NiW6-Tris-CH2OH is the first example of a covalently modified Anderson-type heteropolytungstate. The hydrated sodium tetramethylammonium salt of NiW6-Tris-CH2OH has been synthesized in good yields and has been extensively characterized in solid state and in solution. Zeta potential measurements performed on [Ni(OH)3W6O18(OCH2)3CCH2OH]4− in the presence of HSA demonstrated its applicability to induce charge inversion. The introduction of tris-ligand to Anderson-type POTs suggests existence of a rich tris-functionalized POT chemistry that will be elucidated in the future. The proposed synthesis strategy opens a route for new multi-functional organic–inorganic hybrid materials for various applications.
The research was funded by the Austrian Science Fund (FWF): P27534. N. I. G. thanks OEAD for a scholarship of the Scholarship Foundation of the Republic of Austria for Postdocs. The authors are grateful to Ing. P. Unteregger for support with the ESI-MS measurements at the MS Centre, Univ. of Vienna. We thank Ao.Uni.-Prof. E. Libowitzky for access to ATR-IR measurements and Ao.Uni.-Prof. C. L. Lengauer for TGA measurements at the Institut für Mineralogie und Kristallographie, Univ. of Vienna. Special thanks to Ao.Univ.-Prof. F. Gabor and Mag. J. Gausterer from the Dep. of Pharmaceutical Technology and Biopharmaceutics, Univ. of Vienna for access to zeta potential measurements. Lastly, the authors wish to thank T. Caldera-Fraile, MSc. for the synthesis of CrW6 and Dr A. Bijelic, Dipl.-Chem. C. Molitor, A. Blazevic, MSc. and E. Al-Sayed, MSc. for valuable discussions regarding this work.
Notes and references
- (a) M. T. Pope, Inorganic Chemistry Concepts, Heteropoly and Isopoly Oxometalates, Springer, Berlin, 1983, vol. 8 CrossRef; (b) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef.
- (a) Special Issue: Polyoxometalates, ed. by U. Kortz, T. Liu, Eur. J. Inorg. Chem., 2013, 10–11, 1556 Search PubMed; (b) Y. Ji, L. Huang, J. Hu, C. Streb and Y.-F. Song, Energy Environ. Sci., 2015, 8, 776 RSC.
- (a) J. T. Rhule, C. L. Hill, D. A. Judd and R. F. Schinazi, Chem. Rev., 1998, 98, 327 CrossRef CAS PubMed; (b) B. Hasenknopf, Front. Biosci., 2005, 10, 275 CrossRef CAS PubMed; (c) M. Aureliano and C. André Ohlin, J. Inorg. Biochem., 2014, 137, 123 CrossRef CAS PubMed; (d) M. Aureliano, C. André Ohlin, M. O. Vieira, M. P. M. Marques, W. H. Casey and L. A. E. Batista de Carvalho, Dalton Trans., 2016, 45, 7391 RSC.
- (a) A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed; (b) A. Proust, R. Thouvenot and P. Gouzerh, Chem. Commun., 2008, 1837 RSC; (c) A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605 RSC; (d) M.-P. Santoni, G. S. Hanan and B. Hasenknopf, Coord. Chem. Rev., 2014, 281, 64 CrossRef CAS.
- W. H. Knoth and R. L. Harlow, J. Am. Chem. Soc., 1981, 103, 4265 CrossRef CAS.
- (a) D. Li, J. Song, P. Yin, S. Simotwo, A. J. Bassler, Y. Y. Aung, J. E. Roberts, K. I. Hardcastle, C. L. Hill and T. Liu, J. Am. Chem. Soc., 2011, 133, 14010 CrossRef CAS PubMed; (b) A. Bayaguud, J. Zhang, R. N. N. Khan, J. Hao and Y. Wei, Chem. Commun., 2014, 50, 13150 RSC; (c) H. Sartzi, H. N. Miras, L. Vila-Nadal, D.-L. Long and L. Cronin, Angew. Chem., Int. Ed., 2015, 54, 15488 CrossRef CAS PubMed; (d) H. Zeng, G. R. Newkome and C. L. Hill, Angew. Chem., Int. Ed., 2000, 39, 1772 CAS; (e) C. P. Pradeep, D.-L. Long, G. N. Newton, Y.-F. Song and L. Cronin, Angew. Chem., Int. Ed., 2008, 47, 4460 CrossRef; (f) C. P. Pradeep, M. F. Misdrahi, F.-Y. Li, J. Zhang, L. Xu, D.-L. Long, T. Liu and L. Cronin, Angew. Chem., Int. Ed., 2009, 48, 8309 CrossRef CAS PubMed; (g) C. P. Pradeep, F.-Y. Li, C. Lydon, H. N. Miras, D.-L. Long, L. Xu and L. Cronin, Chem. – Eur. J., 2011, 17, 7472 CrossRef CAS PubMed.
- (a) B. Hasenknopf, R. Delmont, P. Herson and P. Gouzerh, Eur. J. Inorg. Chem., 2002, 1081 CrossRef CAS; (b) P. R. Marcoux, B. Hasenknopf, J. Vaissermann and P. Gouzerh, Eur. J. Inorg. Chem., 2003, 2406 CrossRef CAS; (c) Y.-F. Song, D.-L. Long, S. E. Kelly and L. Cronin, Inorg. Chem., 2008, 47, 9137 CrossRef CAS PubMed; (d) C.-G. Lin, W. Chen, D.-L. Long, L. Cronin and Y.-F. Song, Dalton Trans., 2014, 43, 8587 RSC; (e) H. Ai, Y. Wang, B. Li and L. Wu, Eur. J. Inorg. Chem., 2014, 2766 CrossRef CAS; (f) A. Blazevic, E. Al-Sayed, A. Roller, G. Giester and A. Rompel, Chem. – Eur. J., 2015, 21, 4762 CrossRef CAS PubMed; (g) J. Zhang, Z. Liu, Y. Huang, J. Zhang, J. Hao and Y. Wei, Chem. Commun., 2015, 51, 9097 RSC; (h) J. Zhang, Q. Li, M. Zeng, Y. Huang, J. Zhang, J. Hao and Y. Wei, Chem. Commun., 2016, 52, 2378 RSC; (i) E. Al-Sayed, A. Blazevic, A. Roller and A. Rompel, Chem. – Eur. J., 2015, 21, 17800 CrossRef CAS PubMed; (j) S. Vanhaecht, J. Jacobs, L. Van Meervelt and T. N. Parac-Vogt, Dalton Trans., 2015, 44, 19059 RSC; (k) Y. Ji, J. Hu, L. Huang, W. Chen, C. Streb and Y.-F. Song, Chem. – Eur. J., 2015, 21, 6469 CrossRef CAS PubMed; (l) L. Huang, J. Hu, Y. Ji, C. Streb and Y.-F. Song, Chem. – Eur. J., 2015, 21, 18799 CrossRef CAS PubMed.
- A. Blazevic and A. Rompel, Coord. Chem. Rev., 2016, 307, 42 CrossRef CAS.
- S.-T. Zheng, J. Zhang, X.-X. Li, W.-H. Fang and G.-Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102 CrossRef CAS PubMed.
- (a) S. Bareyt, S. Piligkos, B. Hasenknopf, P. Gouzerh, E. Lacôte, S. Thorimbert and M. Malacria, J. Am. Chem. Soc., 2005, 127, 6788 CrossRef CAS PubMed; (b) C. R. Mayer, C. Roch-Marchal, H. Lavanant, R. Thouvenot, N. Sellier, J.-C. Blais and F. Sécheresse, Chem. – Eur. J., 2004, 10, 5517 CrossRef CAS PubMed; (c) C. R. Mayer, I. Fournier and R. Thouvenot, Chem. – Eur. J., 2000, 6, 105 CrossRef CAS PubMed; (d) G. Sazani, M. H. Dickman and M. T. Pope, Inorg. Chem., 2000, 39, 939 CrossRef CAS PubMed.
- (a) S. G. Mauracher, C. Molitor, R. Al-Oweini, U. Kortz and A. Rompel, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2014, F70, 263 CrossRef PubMed; (b) S. G. Mauracher, C. Molitor, R. Al-Oweini, U. Kortz and A. Rompel, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, D70, 2301 CrossRef PubMed; (c) A. Bijelic, C. Molitor, S. G. Mauracher, R. Al-Oweini, U. Kortz and A. Rompel, ChemBioChem, 2015, 16, 233 CrossRef CAS PubMed; (d) A. Bijelic and A. Rompel, Coord. Chem. Rev., 2015, 299, 22 CrossRef CAS PubMed; (e) C. Molitor, S. G. Mauracher and A. Rompel, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2015, F71, 746 CrossRef PubMed; (f) C. Molitor, S. G. Mauracher and A. Rompel, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E1806 CrossRef CAS PubMed.
- G. M. Rozantsev, S. V. Radio, N. I. Gumerova, V. N. Baumer and O. B. Shishkin, J. Struct. Chem., 2009, 50, 296 CrossRef CAS.
- P. Wu, Z. Xiao, J. Zhang, J. Hao, J. Chen, P. Yin and Y. Wie, Chem. Commun., 2011, 47, 5557 RSC.
- N. I. Gumerova, N. A. Melnik, G. M. Rozantsev, V. N. Baumer and S. V. Radio, J. Struct. Chem., 2015, 56, 926 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, synthesis details, characterization data, including IR, ESI-MS, TGA, elemental analysis and X-ray crystallography with CIF file. CCDC 1477611. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04326g |
| ‡ On leave from: Department of Inorganic Chemistry, Donetsk National University, 21021 Vinnytsia, Ukraine. E-mail: n.gumerova@donnu.edu.ua |
| This journal is © The Royal Society of Chemistry 2016 |