Cooperative Lewis acidity in borane-substituted fluorophosphonium cations†
Abstract
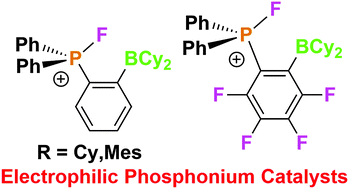
A series of aryl-difluorophosphoranes are converted to give fluorophosphonium salts [Ph2PF(o-C6X4BR2)]+ (X = H, F; R = Cy, Mes). The proximity of the two weakly Lewis acidic fluorophosphonium and borane moieties results in enhanced Lewis acid catalytic reactivity.

 Please wait while we load your content...
Please wait while we load your content...