Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of imidazolinium salts and N-heterocyclic olefins for the synthesis of anionic and neutral tungsten imido alkylidene complexes†
Dominik A.
Imbrich
a,
Wolfgang
Frey
b,
Stefan
Naumann
*a and
Michael R.
Buchmeiser
*ac
aInstitute of Polymer Chemistry, University of Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany. E-mail: michael.buchmeiser@ipoc.uni-stuttgart.de; stefan.naumann@ipoc.uni-stuttgart.de
bInstitute of Organic Chemistry, University of Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
cInstitute of Textile Chemistry and Chemical Fibers, Körschtalstraße 26, D-73770 Denkendorf, Germany
First published on 4th April 2016
Abstract
The synthesis, single crystal X-ray structure and activity in olefin metathesis of novel anionic tungsten imido alkylidene complexes [1,3-bis-(2,4,6-trimethylphenyl)imidazolinium]+ [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2,5-Me2Pyr)2Cl]−, [1,3-bis-(2,4,6-trimethylphenyl)imidazolinium]+ [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2,5Me2Pyr)2(OC6F5)]−, and [1,3-bis-(2,6-diisopropylphenyl)imidazolinium]+ [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2,5-Me2Pyr)Cl2]− are reported. Additionally, the first example of a bis(N-heterocyclic olefinium) alkylidene tungstate, W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2-methylene-1,3,4,5-tetramethyl-imidazoline)2(OTf)2, is described, including preparation, crystal structure and catalytic activity.
Recently, our group reported on the synthesis of cationic tungsten oxo alkylidene N-heterocyclic carbene (NHC) and neutral as well as cationic molybdenum imido alkylidene NHC complexes.1–4 These complexes show high activity in olefin metathesis reactions. Importantly, their considerable reactivity is paired with substantial tolerance against functional groups, which previously presented a major complication for widespread application of tungsten- and molybdenum-derived metathesis catalysts.5 In view of these first promising results, we aimed for an extension of NHC-coordination to tungsten imido alkylidene based systems. Additionally, a newly emerging class of strongly electron-donating compounds, N-heterocyclic olefins (NHOs),6 presented itself as a potential alternative to NHCs and was likewise applied in tungsten imido complexes. The compounds resulting from these research efforts will be discussed in the following.
In search of novel W-imido alkylidene NHC complexes, the bispyrrolide precursor7 [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2,5-Me2NC4H2)2] (1) was reacted with 1,3-(2,4,6-trimethylphenyl)-imidazolin-2-ium chloride (Scheme 1). This procedure was expected to work via in situ deprotonation of the NHC precursor by pyrrolide, in analogy to similar findings of Schrock and co-workers, who introduced alkoxide ligands by reacting alcohols with pyrrolide-bearing complexes.8 This exchange of ligands was envisioned to serve as an elegant means to introduce an NHC ligand while keeping the electron count constant. Surprisingly, this reaction did not yield the desired W-imido alkylidene NHC complexes; instead, the corresponding anionic W-imido chloro alkylidene bispyrrolide was formed in high yield. While thwarting the attempts to introduce NHCs, this rather unexpected chloro-transfer offered access to anionic imido alkylidene complexes. Reports on such compounds are very rare and include only a limited number of examples,9–11 like Winter's [W(CHPh)(CO)2(η-C5H5)−]12 or [Cp*Mo(NO)(CH2SiMe3)-(CHSiMe3)]2[Li2(THF)3]13 as described by Legzdins. So far, all these complexes have been solely used as intermediates for the synthesis of non-ionic compounds and no activity in olefin metathesis reactions of these complexes was reported. To exploit the above-described reaction in a systematic manner, complexes 2–4 were prepared as outlined in Scheme 1. Starting from the literature-known precursor complex 1,5,7 compound 2 was conveniently obtained in high yield by adding a suspension of 1,3-bis(2,4,6-trimethylphenyl)imidazolinium chloride to a stirred solution of complex 1 in benzene. Analytically pure samples could be crystallized from CH2Cl2 by adding small amounts of pentane. Complex 2 crystallizes in the monoclinic space group P21/c, a = 2168.65(19) pm, b = 1887.37(16) pm, c = 1610.05(13) pm, α = 90°, β = 106.667(3)°, γ = 90°, Z = 4. Relevant bond lengths and angles are summarized in Fig. 1.
In the solid state, complex 2 adopts a slightly distorted square pyramidal (SP) configuration with the alkylidene forming the apex. The tungsten alkylidene bond is 190.2(5) pm and the pyrrolides have almost the same distance to the metal centre (213.6(5) and 215.0(5) pm), respectively. Both, bond angles and bond lengths, indicate an η1-coordination of both pyrrolides. In the solid state, the alkylidene is observed in a syn configuration, which correlates with solution NMR data (JC–H = 121.63 Hz (CD2Cl2)) at −20 °C.
Nonetheless, broad signals observed in 1H NMR for the alkylidene, pyrrolides and the 2-propyl groups of the imido ligand (Fig. S1 and S2, ESI†) suggest a dynamic structure in solution at room temperature.
Importantly, further functionalization of 2 could be easily achieved. Reaction with one equivalent of lithium pentafluorophenoxide leads to the formation of 3 in high yield (Scheme 1), analytically pure samples of which were again crystallized from CH2Cl2 by adding small amounts of pentane. Complex 3 crystallizes in the monoclinic space group P21/c, a = 1124.44(3) pm, b = 2612.67(5) pm, c = 2061.54(4) pm, α = 90°, β = 94.9910(10), γ = 90°, Z = 4. Relevant bond lengths and angles are summarized in Fig. 2. Similar to 2, in the solid state complex 3 adopts a distorted square pyramidal configuration again with the alkylidene forming the apex. The tungsten alkylidene bond is 188.9(2) pm, which is comparable to the bond in 2. Again, the pyrrolides have almost the same distance to the metal center (212.74(18) and 213.48(18) pm), and η1-bonding of the pyrrolide can be deducted from bond angles and bond lengths. In the solid state the alkylidene is observed in a syn configuration, which correlates with solution NMR data (JC–H = 123.0 Hz (CD2Cl2)). The shorter bond lengths of the other ligands are believed to be a result of the more pronounced electron-withdrawing character of the pentafluorophenoxide compared to the chloro-ligand. Unlike 2, compound 3 shows a sharp alkylidene and pyrrolide signal in proton NMR (S3), accompanied by a splitting of the 2-propyl groups at the imido ligand, which is indicative of the hindered rotation of the latter.
Notably, the reaction of the dipyrrolyl precursor [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(2,5-Me2Pyr)2] (1) with two equivalents of 1,3-(2,6-diisopropylphenyl)imidazolin-2-ium chloride leads to the formation of the anionic W-imido dichloro alkylidene pyrrolide complex 4, underlining that this way to generate anionic alkylidenes is not limited to a “magic pair”, but might be achieved with a number of educts. Complex 4 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 1055.91(3) pm, b = 1362.70(5) pm, c = 2123.26(7) pm, α = 86.879(2)°, β = 81.476(2)°, γ = 88.576(2)°, Z = 2. Relevant bond lengths and angles are summarized in Fig. 3.
, a = 1055.91(3) pm, b = 1362.70(5) pm, c = 2123.26(7) pm, α = 86.879(2)°, β = 81.476(2)°, γ = 88.576(2)°, Z = 2. Relevant bond lengths and angles are summarized in Fig. 3.
As a solid, compound 4 adopts a distorted SP-configuration with the alkylidene forming the apex. The tungsten alkylidene bond is 188.6(2) pm, which is comparable to the corresponding one in complexes 2 and 3. The pyrrolide has a shorter distance to the metal center (210.4(2) pm). η1-Bonding of the pyrrolide is again suggested by characteristic values of bond lengths and bond angles. As found for 2 and 3, the alkylidene is observed in a syn configuration, which correlates with solution NMR data (JC–H = 122.06 Hz (CD2Cl2)).
It should be noted that analogous reactions with 1,3-dimethylimidazolium iodide or [1,3-bis-(2,4,6-trimethylphenyl)imidazolinium] tetrafluoroborate did not result in the formation of the corresponding anionic complexes, most probably as a consequence of the decreased nucleophilicity of iodide and tetrafluoroborate, respectively. Use of iodide resulted in no reaction at all, while application of the tetrafluoroborate salt resulted in complete decomposition of the metal complex.
The anionic tungsten alkylidene complexes 2, 3 and 4 were subjected to a set of simple olefin metathesis reactions to test for their activity and functional group tolerance in ring-closing metathesis (RCM), homometathesis (HM), self-metathesis (SM) and cross-metathesis (CM) reactions. Generally, the activity of group 6 metal alkylidenes in olefin metathesis reactions strongly depends on the polarization of the metal–alkylidene bond and the electrophilic character of the metal center.7 In this regard, no metathetical activity of anionic compounds 2–4 can be expected. Surprisingly, 2–4 show a low but significant propensity to catalyze olefin metathesis reactions (Table S1, ESI†): in the RCM of diallydiphenylsilane and the HM of terminal alkenes such as 1-hexene and 1-octene turnover numbers (TONs) in the range of 50 to 410 could be achieved. Potential “activation” of complexes 2 and 4 by abstraction of chloride (using AlCl3) has failed so far, but remains under investigation since this represents an attractive access to latent polymerization systems.14,15
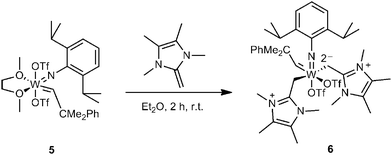
In view of the above-described findings, attention was then shifted to NHOs. This class of very electron-rich and polarized olefins is currently receiving increasing interest.6 The charge separation characterizing the double bond in NHOs can be strong enough to allow for the formation of CO2-adducts;16 likewise, their considerable nucleophilicity and basicity were recently put to use in the first NHO-mediated organopolymerizations.17,18 A limited number of NHO–metal complexes are also known,19–23 and remarkably, it was found that NHOs can confer more electron density onto the metal centre than the traditionally strongly donating NHCs.24 Furthermore, Rivard and co-workers have demonstrated that NHOs can be considered “soft” donors, with higher σ-donor propensity but less bonding strength than NHCs.25 In summary, this recommends NHOs for deeper investigations in organometallic chemistry, particularly if considered what penetrating impact NHCs have had in this field. NHO alkylidenes have not been described before, and we were curious as to if it was possible to generate them by direct reaction with a precursor complex. Selecting 2-methylene-1,3,4,5-tetramethylimidazoline (Scheme 2) as a sterically non-hindered and synthetically well accessible, strongly polar NHO20,26 and W(CHCMe2Ph)(N-2,6-iPr2C6H3)(OTf)2(dme)5 (5) as precursor alkylidene, it was found that direct reaction in diethyl ether using two equivalents of the NHO cleanly afforded complex 6. This compound bears two NHO-derived ligands; reactions using only one equivalent of NHO resulted in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixtures of educt 5 and compound 6. Analytically pure samples were crystallized from CH2Cl2 by adding small amounts of pentane. Complex 6 crystallizes in the triclinic space group P
50 mixtures of educt 5 and compound 6. Analytically pure samples were crystallized from CH2Cl2 by adding small amounts of pentane. Complex 6 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 117.112(7) pm, b = 203.641(13) pm, c = 219.201(14) pm, α = 103.305(3)°, β = 97.459(2)°, γ = 100.855(3)°, Z = 4. Relevant data are summarized in Fig. 4.
, a = 117.112(7) pm, b = 203.641(13) pm, c = 219.201(14) pm, α = 103.305(3)°, β = 97.459(2)°, γ = 100.855(3)°, Z = 4. Relevant data are summarized in Fig. 4.
NHO alkylidene 6 adopts a slightly distorted octahedral configuration with one triflate and the imido ligand forming the apices. The tungsten alkylidene bond is 192.02(2) pm, which is comparable to the reported bistriflate NHC complexes.1 The NHO ligands are in trans-position (153.42(9)°) to each other and sp3 hybridized at the methylene moiety, which indicates full charge separation and formation of a covalent bond to the metal centre. In the solid state the alkylidene is syn to the imido ligand (JC–H = 114.06 ppm). With 227.04(16) pm and 240.05(16) pm the triflate–metal bonds are very dissimilar and weak. This is compounded by 19F NMR, which displays a signal at −78.4 ppm, suggesting a weakly bound triflate in solution that is close in chemical shift to free triflate (see Table S2 for a comparison of different alkylidene triflate complexes with regard to the chemical shift and bond length, ESI†).1
Complex 6 was also subjected to a set of basic olefin metathesis reactions (Table S1, ESI†). The RCM of diallydiphenylsilane and 1-hexene led to conversion with TONs in the range of 140 to 250, but the reactivity was generally low, most probably as a result of the steric (and electronic) saturation of complex 6. Since NHOs are easily modulated, this situation is expected to improve soon and investigations are ongoing in our group.
In conclusion, anionic tungsten imido alkylidene complexes have been reported and fully characterized, accessible via a very simple route using imidazolinium chlorides. Additionally, an alkylidene NHO complex could be synthesized, which represents the first of a class of potentially very promising catalysts for metathesis reactions.
This work was supported by the Deutsche Forschungs-gemeinschaft (DFG, BU 2174/19-1).
References
- M. R. Buchmeiser, S. Sen, J. Unold and W. Frey, Angew. Chem., Int. Ed., 2014, 53, 9384–9388 CrossRef CAS PubMed.
- R. Schowner, W. Frey and M. R. Buchmeiser, J. Am. Chem. Soc., 2015, 137, 6188–6191 CrossRef CAS PubMed.
- S. Sen, R. Schowner, D. A. Imbrich, W. Frey, M. Hunger and M. R. Buchmeiser, Chem. – Eur. J., 2015, 21, 13778–13787 CrossRef CAS PubMed.
- M. Pucino, V. Mougel, R. Schowner, A. Fedorov, M. R. Buchmeiser and C. Copéret, Angew. Chem., Int. Ed., 2016, 55, 4300–4302 CrossRef CAS PubMed.
- R. R. Schrock, R. T. DePue, J. Feldman, K. B. Yap, D. C. Yang, W. M. Davis, L. Park, M. DiMare and M. Schofield, Organometallics, 1990, 9, 2262–2275 CrossRef CAS.
- R. D. Crocker and T. V. Nguyen, Chem. – Eur. J., 2016, 22, 2208–2213 CrossRef CAS PubMed.
- R. R. Schrock and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4592–4633 CrossRef CAS PubMed.
- R. R. Schrock, Chem. Rev., 2009, 109, 3211–3226 CrossRef CAS PubMed.
- M. T. Jan, S. Sarkar, S. Kuppuswamy, I. Ghiviriga, K. A. Abboud and A. S. Veige, J. Organomet. Chem., 2011, 696, 4079–4089 CrossRef CAS.
- M. Rudler-Chauvin and H. Rudler, J. Organomet. Chem., 1981, 212, 203–210 CrossRef CAS.
- S. Dovesi, E. Solari, R. Scopelliti and C. Floriani, Angew. Chem., Int. Ed., 1999, 38, 2388–2391 CrossRef CAS.
- J. E. Muir, A. Haynes and M. J. Winter, Chem. Commun., 1996, 1765–1766 RSC.
- P. Legzdins and S. F. Sayers, Organometallics, 1996, 15, 3907–3909 CrossRef CAS.
- S. Monsaert, A. L. Vila, R. Drozdzak, P. Van Der Voort and F. Verpoort, Chem. Soc. Rev., 2009, 38, 3360–3372 RSC.
- S. Naumann and M. R. Buchmeiser, Macromol. Rapid Commun., 2014, 35, 682–701 CrossRef CAS PubMed.
- Y.-B. Wang, Y.-M. Wang, W.-Z. Zhang and X.-B. Lu, J. Am. Chem. Soc., 2013, 135, 11996–12003 CrossRef CAS PubMed.
- S. Naumann, A. W. Thomas and A. P. Dove, ACS Macro Lett., 2016, 5, 134–138 CrossRef CAS.
- S. Naumann, A. W. Thomas and A. P. Dove, Angew. Chem., Int. Ed., 2015, 54, 9550–9554 CrossRef CAS PubMed.
- N. Kuhn, H. Bohnen, D. Bläser and R. Boese, Chem. Ber., 1994, 127, 1405–1407 CrossRef CAS.
- H. Schumann, M. Glanz, J. Winterfeld, H. Hemling, N. Kuhn, H. Bohnen, D. Bläser and R. Boese, J. Organomet. Chem., 1995, 493, C14–C18 CrossRef CAS.
- S. M. Ibrahim Al-Rafia, A. C. Malcolm, S. K. Liew, M. J. Ferguson, R. McDonald and E. Rivard, Chem. Commun., 2011, 47, 6987–6989 RSC.
- S. Kronig, P. G. Jones and M. Tamm, Eur. J. Inorg. Chem., 2013, 2301–2314 CrossRef CAS.
- M. Iglesias, A. Iturmendi, P. J. Sanz Miguel, V. Polo, J. J. Perez-Torrente and L. A. Oro, Chem. Commun., 2015, 51, 12431–12434 RSC.
- A. Fürstner, M. Alcarazo, R. Goddard and C. W. Lehmann, Angew. Chem., Int. Ed., 2008, 47, 3210–3214 CrossRef PubMed.
- K. Powers, C. Hering-Junghans, R. McDonald, M. J. Ferguson and E. Rivard, Polyhedron, 2016 DOI:10.1016/j.poly.2015.07.070.
- N. Kuhn, H. Bohnen, J. Kreutzberg, D. Blaser and R. Boese, Chem. Commun., 1993, 1136–1137 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and details of single crystal X-ray structures of 2, 3, 4 and 6. CCDC 1456761–1456764. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc02483a |
| This journal is © The Royal Society of Chemistry 2016 |