Regioselective construction of diverse and multifunctionalized 2-hydroxybenzophenones for sun protection by indium(III)-catalyzed benzannulation†
Hongyun
Cai
,
Likai
Xia
 and
Yong Rok
Lee
*
and
Yong Rok
Lee
*
School of Chemical Engineering, Yeungnam University, Gyeongsan 712-749, Republic of Korea. E-mail: yrlee@yu.ac.kr; Fax: +82-53-810-4631; Tel: +82-53-810-2529
First published on 29th April 2016
Abstract
Diverse and functionalized 2-hydroxybenzophenone derivatives were synthesized efficiently in good to excellent yield via the highly regioselective indium(III)-catalyzed [2+2+2] benzannulation of 3-formylchromones with β-enamino esters or β-enamino ketones. This benzannulation reaction proceeds via a domino Michael/retro-Michael/6π-electrocyclization/deformylation reaction. In addition, 2-hydroxybenzophenones were also prepared by the indium(III)-catalyzed [4+2] benzannulation reaction between 3-substituted chromen-4-ones and β-enamino esters or β-enamino ketones. Furthermore, the effects of substituents and π conjugation on the characteristics of the UV-Vis spectrum of synthesized 2-hydroxybenzophenones were examined. Compound 10s exhibited higher sun protection activity than oxybenzone which is used in most popular sunscreens.
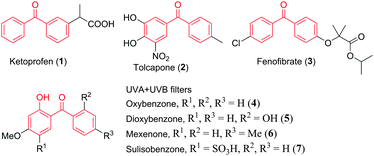
Benzophenone is an important structural motif, which is present in many biologically active natural products as well as pharmaceuticals.1 These benzophenone-based molecules have a wide range of pharmacological properties, such as antioxidant,2 antityrosinase,3 antibacterial,4 antifungal,5 antiviral,6 cytotoxicity,7 anti-HIV,8 anticancer,9 anti-hepatitis B,7 and Mycobacterium tuberculosis (Mtb) pantothenate synthetase inhibitory activities.10Fig. 1 shows some commercially available drugs and sun-protection materials that contain a benzophenone moiety. For example, ketoprofen (1) is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic effects.11 Tolcapone (2, brand name Tasmar) is used to treat Parkinson's disease.12 Fenofibrate (3), which is marketed as Tricor, is used mainly to reduce the cholesterol levels in patients at risk of cardiovascular disease.13 In addition, benzophenones are also used widely as photosensitizers and represent one of the most important substance classes in photochemistry.14 In particular, UV-A/B filters, such as 2-hydroxybenzophenones (4–7) are the most commonly used active ingredients in sun protection products (e.g. sunscreens) that prevent ultraviolet light from passing through the skin.15
Owing to their important bioactivities and pharmaceutical applications, a number of synthetic approaches for the preparation of benzophenones and their derivatives have been developed. The classical strategies involve mainly Friedel–Crafts acylations16 or reactions of aryllithium or magnesium reagents with aldehydes followed by oxidation.17 Other representative examples include palladium-catalyzed cross coupling reaction of arylboronic acids with carboxylic acids,18 rhodium-catalyzed reaction of potassium trifluoro(organo)borates with arylaldehydes,19 iron-catalyzed carbonylative Suzuki reactions,20 and nickel-catalyzed addition of arylboronic acids to nitriles21 (Scheme 1).
Although many methods have been developed for the synthesis of benzophenones through typical procedures and transition-metal catalyzed reactions, there are no examples for the direct construction of 2-hydroxybenzophenones utilizing indium(III)-catalyzed benzannulation. For 2-hydroxybenzophenones, Me3SiOTf-catalyzed domino reaction of 1-aryl-1,3-bis(silyloxy)buta-1,3-dines and 3-formylchromones via formal [3+3] cycloaddition has been reported.22 On the other hand, environmentally benign and efficient approaches for the direct construction of highly functionalized and diverse 2-hydroxybenzophenones from commercially available starting materials are still desirable. In this regard, benzannulation is one of the most important and valuable strategies for the construction of desirable aromatic rings, which prompt us to develop new approaches for the synthesis of 2-hydroxybenzophenones. Over the previous few years, a range of benzannulation reactions have been developed for forming highly substituted benzenes.23 Recently, the transition-metal-free multicomponent benzannulation reactions of sodium sulfonate with 4-bromocrotonate and α,β-unsaturated aldehydes24 or 1,3-dicarbonyls with nitroalkenes and dialkyl acetylenedicarboxylates have been described for the preparation of diverse benzene rings.25 To the best of our knowledge, there is no report on multicomponent benzannulation between 4-oxo-4H-chromene-3-carbaldehydes and β-enamino esters or β-enamino ketones. We became interested in the benzannulation reactions as a powerful means of synthesizing a variety of aromatics and heteroaromatics, such as biaryls,26 anthraquinones and tetracenediones,27 carbazoles,28 and pyridines.29 As part of an ongoing study in this area, this paper reports the direct one-pot construction of diverse 2-hydroxybenzophenones by the indium(III)-catalyzed benzannulation of readily available 4-oxo-4H-chromene-3-carbaldehydes with β-enamino esters or β-enamino ketones (Scheme 2, eqn (1)). As an application of this protocol, this paper also describes novel benzannulation between 3-substituted chromenones and β-enamino esters or β-enamino ketones for producing a range of 2-hydroxybenzophenones (Scheme 2, eqn (2)).
 | ||
| Scheme 2 Our strategy for the construction of diverse and various 2-hydroxybenzophenones by [2+2+2] and [4+2] benzannulation. | ||
To produce 2-hydroxybenzophenones, we first examined the reaction of 3-formylchromone (8a) with 9a in the presence of several catalysts. The results are depicted in Table 1. No products were produced in the absence of a catalyst (entry 1). The initial attempts with rare-earth metal triflates, such as Hf(OTf)4, Y(OTf)3, or Yb(OTf)3, in acetonitrile at room temperature for 24 h, did not provide any of the desired products, but the reaction materials decomposed (entries 2–4). Other metal triflates were also screened, and the best yield (85%) was achieved with 5 mol% of In(OTf)3 in acetonitrile at room temperature for 8 h (entries 5–7). Decreasing In(OTf)3 to 2 mol% or increasing In(OTf)3 to 10 mol% lowered the yield of 10a (entries 8 and 9). With other indium catalysts, the yield of 10a did not improve (entries 10–13). In other non-/low-polar solvents, 10a was produced in 10–20% yield (entries 14–17), whereas 10a was produced in polar solvents such as ethanol and dimethylsulfoxide in 75 and 65% yield, respectively (entries 18 and 19). On the other hand, 10a was not obtained in water (entry 20). Among the tested Lewis acids, In(OTf)3 exhibited superior catalytic efficiency to other Lewis acids, which would be attributed to their charge density.30 Furthermore, the recently reported density functional theory (DFT) calculations showed that low LUMO levels of In(OTf)3 would be responsible for the formation of stable coordination intermediate with carbonyl compounds,31a and the strong affinities with carbonyl groups were also observed in other In(OTf)3-catalyzed reactions.31b
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 8a (0.5 mmol), 9a (1.1 mmol), solvent (5.0 mL), room temperature. b Isolated yield. | ||||
| 1 | — | MeCN | 24 | 0 |
| 2 | Hf(OTf)4 (5) | MeCN | 24 | 0 |
| 3 | Y(OTf)3 (5) | MeCN | 24 | 0 |
| 4 | Yb(OTf)3 (5) | MeCN | 24 | 0 |
| 5 | Cu(OTf)2 (10) | MeCN | 15 | 25 |
| 6 | AgOTf (10) | MeCN | 12 | 75 |
| 7 | ln(OTf)3 (5) | MeCN | 8 | 85 |
| 8 | ln(OTf)3 (2) | MeCN | 8 | 72 |
| 9 | ln(OTf)3 (10) | MeCN | 8 | 83 |
| 10 | ln2O3 (30) | MeCN | 24 | Trace |
| 11 | ln(acac)3 (30) | MeCN | 15 | 50 |
| 12 | lnCl3 (10) | MeCN | 15 | 20 |
| 13 | lnBr3 (10) | MeCN | 15 | 25 |
| 14 | ln(OTf)3 (5) | CH2Cl2 | 15 | 15 |
| 15 | ln(OTf)3 (5) | 1,4-Dioxane | 15 | 10 |
| 16 | In(OTf)3 (5) | Toluene | 15 | 20 |
| 17 | ln(OTf)3 (5) | THF | 15 | 14 |
| 18 | ln(OTf)3 (5) | EtOH | 10 | 75 |
| 19 | ln(OTf)3 (5) | DMSO | 12 | 65 |
| 20 | ln(OTf)3 (5) | H2O | 24 | 0 |
Under the optimized conditions, this study further explored the generality of this benzannulation reaction employing different 3-formylchromones 8a–8l (Fig. S1, ESI†) with various β-enamino esters 9a–9d (Table 2). The reactions of 8a with β-enamino esters 9b–9d bearing ethyl, prenyl, or benzyl group on the ester moiety provided the corresponding products 10b–10d in 92, 81, and 78% yield, respectively. Next, the reactions of 8b bearing an electron-donating group on the benzene ring with 9a, 9c, or 9d in MeCN at room temperature for 6–18 h afforded 2-hydroxybenzophenones 10e–10g in 82, 72, and 75% yield, respectively. Similarly, reactions of 8c–8e bearing other electron-donating groups with β-enamino esters 9a–9d provided the corresponding 2-hydroxybenzophenones 10h–10l in 68–78% yield. Further reactions of 3-formylchromones bearing electron-withdrawing groups on the benzene ring were also successful. A combination of 8f–8i with β-enamino esters 9a–9d gave the desired products 10m–10s in 80–91% yield. With 8j or 8k bearing two substituents on the benzene ring, products 10t–10v were produced in 81, 75, and 78% yield, respectively. Interestingly, with 8l, the desired products 10w and 10x were formed in 75 and 78% yield, respectively.
The scope of the reaction was extended further by employing 3-formylchromones 8a, 8d, 8f, or 8m and other β-enamino ketones 11a–11c as shown in Table 3. When 8a, 8d, 8f, or 8m were treated with 11a under optimized reaction conditions, products 12a–12d were formed in 75, 70, 83, and 75% yield, respectively. Furthermore, the treatment of 8d or 8m with 11b or 11c containing substituted acetophenone groups also provided the desired products 12e and 12f in 85 and 82% yield, respectively.
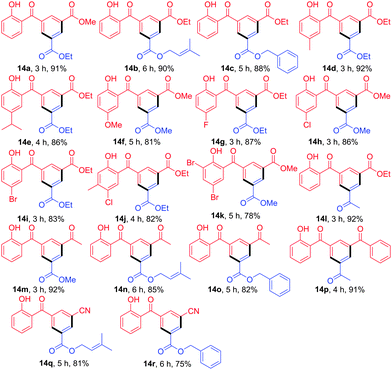
This benzannulation reaction was further applied to various 3-functionalized chromen-4-ones for the construction of diverse 2-hydroxybenzophenones (Table 4). The reaction of 13a with 9b at room temperature for 3 h provided 14a in 91% yield, whereas the treatment of 13b with 9c or 9d afforded the desired products 14b and 14c in 90 and 88% yield, respectively. With chromenyl acrylates 13c–13e bearing electron-donating groups on the chromen-4-one ring, products 14d–14f were produced in 92, 86, and 81% yield, respectively. Similarly, reactions of other chromenyl acrylates 13f–13h with bearing electron-withdrawing groups on the chromen-4-one ring provided the desired products 14g, 14h, and 14i in 87, 86, and 83% yield, respectively. Combination of chromenylacrylates 13i or 13j bearing two substituents on the benzene ring with β-enamino esters afforded the products 14j and 14k in 82 and 78% yields. Further reaction of 13a with β-enamino ketone 11a gave product 14l in 92% yield. In the cases of chromenones 13k and 13l bearing 3-oxobutenyl or chalcone group at the 3-position, the desired products 14m–14p were formed in 82–92% yield. Importantly, reactions of 13m bearing an acrylonitrile group at the 3-position instead of carbonyl groups with the corresponding β-enamino esters provided products 14q and 14r in 81 and 75% yield, respectively.
Based on the observed products and control experiments (Scheme S1, ESI†), the mechanism for the formation of 10a can be explained as shown in Scheme 3. The 3-formylchromone 8a first gives complex A in the presence of In(OTf)3 catalyst to enhance the reactivity of Michael-type addition,32 which is followed by nucleophilic attack by 9a to afford intermediate B. A subsequent retro-Michael reaction of intermediate B followed by double bond migration through deprotonation affords another intermediate C, which undergoes the conjugate addition by 9a to give intermediate D. The intramolecular cyclization of enolate motif with iminium ion in D forms a six-membered ring intermediate E, which further undergoes elimination reactions of dimethylamine and dimethylformamide to afford the final product 10a.
2-Hydroxybenzophenones offer photoprotection by virtue of their high UV absorption cross sections and high internal conversion efficiencies.14,15 The UV absorption results in electronic excitation, followed by efficient internal conversion (IC) and subsequent vibrational relaxation (VR) to the ground state, and thus most of the absorbed energy is safely dissipated as heat.33 These properties of 2-hydroxybenzophenones have tremendous commercial potential in sunscreens. The synthesized 2-hydroxybenzophenones offered protection activity against UVA and UVB with similar sun protection factors (SPFin vitro, range: 1.10–2.26, at 25 μM, Table S2, ESI†) relative to oxybenzone (OBZ, SPFin vitro = 2.12, at 25 μM), a well-known organic UVA/UVB absorber that is used in most popular sunscreens.15 Among these, compound 10s showed higher activity than oxybenzone. To gain a better understanding of the electronic excitation mechanism, the UV absorption spectra of 10a was investigated with the PCM-TD-DFT theoretical scheme using five hybrid functionals (B3LYP, B3PW91, PBE0, M062X, and CAM-B3LYP) and taking into consideration the ethanol solvation effect.33,34 From the results listed in Table S3 (ESI†), the B3PW91 functional appears to be the more accurate hybrid for λmax evaluation whereas B3LYP and PBE0 would be functionals of choice for λmax calculations. The first low-energy excitation of 10a is a dominant HOMO→LUMO transition and shifts with changes in the hydroxyl group position (Fig. S4, ESI†). Further investigations of the substrate effects showed that electron-donating groups on the C-3 and C-4 would cause the hypsochromic shifts, but the electron-donating groups on C-5 and enhanced aromatic conjugation would induce the bathochromic shifts (Fig. S2, ESI†).
An efficient and highly regioselective indium(III)-catalyzed [2+2+2] benzannulation of 3-formylchromones with β-enamino esters or β-enamino ketones was developed for the construction of diverse and functionalized 2-hydroxybenzophenones. In addition, various 2-hydroxybenzophenones were also prepared by the indium(III)-catalyzed [4+2] benzannulation of 3-substituted chromen-4-ones and β-enamino esters or β-enamino ketones. Furthermore, the synthesized compounds can be utilized as important lead compounds for the discovery of new sun-protection materials.
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A2A1A11052391) and the Nano Material Technology Development Program (2012M3A7B4049675).
Notes and references
- S.-B. Wu, C. Long and E. J. Kennelly, Nat. Prod. Rep., 2014, 31, 1158–1174 RSC.
- G. Rodriguez-Fuentes, J. J. Sandoval-Gio, A. Arroyo-Silva, E. Noreña-Barroso, K. S. Escalante-Herrera and F. Olvera-Espinosa, Ecotoxicol. Environ. Saf., 2015, 115, 14–18 CrossRef CAS PubMed.
- J. Wu, X. Hu and L. Ma, J. Enzyme Inhib. Med. Chem., 2011, 26, 449–452 CrossRef CAS PubMed.
- S. E. Ukwueze, P. O. Osadebe and F. B. C. Okoye, Nat. Prod. Res., 2015, 29, 1728–1734 CrossRef CAS PubMed.
- V. Girish, N. F. Khanum, H. D. Gurupadaswamy and S. A. Khanum, Russ. J. Bioorg. Chem., 2014, 40, 330–335 CrossRef CAS.
- R. Liu, L. An, G. Liu, X. Li, W. Tang and X. Chen, Antiviral Res., 2015, 120, 101–111 CrossRef CAS PubMed.
- J. Zhang, L.-l. Fu, M. Tian, H.-q. Liu, J.-j. Li, Y. Li, J. He, J. Huang, L. Ouyang, H.-y. Gao and J.-h. Wang, Bioorg. Med. Chem., 2015, 23, 976–984 CrossRef CAS PubMed.
- X.-D. Ma, X. Zhang, H.-F. Dai, S.-Q. Yang, L.-M. Yang, S.-X. Gu, Y.-T. Zheng, Q.-Q. He and F.-E. Chen, Bioorg. Med. Chem., 2011, 19, 4601–4607 CrossRef CAS PubMed.
- J. McNulty, S. van den Berg, D. Ma, D. Tarade, S. Joshi, J. Church and S. Pandey, Bioorg. Med. Chem. Lett., 2015, 25, 117–121 CrossRef CAS PubMed.
- P. B. Devi, G. Samala, J. P. Sridevi, S. Saxena, M. Alvala, E. G. Salina, D. Sriram and P. Yogeeswari, ChemMedChem, 2014, 9, 2538–2547 CrossRef CAS PubMed.
- T. G. Kantor, Pharmacotherapy, 1986, 6, 93–103 CrossRef CAS PubMed.
- J. Dingemanse, K. Jorga, G. Zurcher, M. Schmitt, G. Sedek, M. D. Prada and P. Van Brummelen, Br. J. Clin. Pharmacol., 1995, 40, 253–262 CrossRef CAS PubMed.
- T. Y. Wong, R. Simó and P. Mitchell, Am. J. Ophthalmol., 2012, 154, 6–12 CrossRef CAS PubMed.
- X. Cai, M. Sakamoto, M. Fujitsuka and T. Majima, Chem. – Eur. J., 2005, 11, 6471–6477 CrossRef CAS PubMed.
- Z. Klimová, J. Hojerová and M. Beranková, Food Chem. Toxicol., 2015, 83, 237–250 CrossRef PubMed.
- X. Shi, W. Kueh, B. Zheng, K.-W. Huang and C. Chi, Angew. Chem., Int. Ed., 2015, 54, 14412–14416 CrossRef CAS PubMed.
- H. Li, R.-Y. Zhu, W.-J. Shi, K.-H. He and Z.-J. Shi, Org. Lett., 2012, 14, 4850–4853 CrossRef CAS PubMed.
- L. J. Gooßen and K. Ghosh, Angew. Chem., Int. Ed., 2001, 40, 3458–3460 CrossRef.
- M. Pucheault, S. Darses and J.-P. Genet, J. Am. Chem. Soc., 2004, 126, 15356–15357 CrossRef CAS PubMed.
- Y. Zhong and W. Han, Chem. Commun., 2014, 50, 3874–3877 RSC.
- Y.-C. Wong, K. Parthasarathy and C.-H. Cheng, Org. Lett., 2010, 12, 1736–1739 CrossRef CAS PubMed.
- M. A. Yawer, I. Hussain, C. Fischer, H. Göerls and P. Langer, Tetrahedron, 2008, 64, 894–900 CrossRef CAS.
- (a) F. Gao and Y. Huang, Adv. Synth. Catal., 2014, 356, 2422–2428 CrossRef CAS; (b) F. Ling, Z. Li, C. Zheng, X. Liu and C. Ma, J. Am. Chem. Soc., 2014, 136, 10914–10917 CrossRef CAS PubMed; (c) A. Wittstock and M. Bäumer, Acc. Chem. Res., 2014, 47, 731–739 CrossRef CAS PubMed.
- P. R. Joshi, S. Undeela, D. D. Reddy, K. K. Singarapu and R. S. Menon, Org. Lett., 2015, 17, 1449–1452 CrossRef CAS PubMed.
- W.-M. Shu, K.-L. Zheng, J.-R. Ma and A.-X. Wu, Org. Lett., 2015, 17, 5216–5219 CrossRef CAS PubMed.
- T. N. Poudel and Y. R. Lee, Org. Lett., 2015, 17, 2050–2053 CrossRef CAS PubMed.
- K. B. Somai Magar, L. Xia and Y. R. Lee, Chem. Commun., 2015, 51, 8592–8595 RSC.
- T. N. Poudel and Y. R. Lee, Chem. Sci., 2015, 6, 7028–7033 RSC.
- H. D. Khanal and Y. R. Lee, Chem. Commun., 2015, 51, 9467–9470 RSC.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Wiley-Interscience, New York, 6th edn, 1999 Search PubMed.
- (a) Y. Koito, K. Nakajima, H. Kobayashi, R. Hasegawa, M. Kitano and M. Hara, Chem. – Eur. J., 2014, 20, 8068–8075 CrossRef CAS PubMed; (b) M. Y. Yoon, J. H. Kim, D. S. Choi, U. S. Shin, J. Y. Lee and C. E. Song, Adv. Synth. Catal., 2007, 349, 1725–1737 CrossRef CAS.
- (a) L. Xia and Y. R. Lee, RSC Adv., 2014, 4, 36905–36916 RSC; (b) L. Xia and Y. R. Lee, Org. Biomol. Chem., 2013, 11, 6097–6107 RSC.
- L. A. Baker, M. D. Horbury, S. E. Greenough, P. M. Coulter, T. N. V. Karsili, G. M. Roberts, A. J. Orr-Ewing, M. N. R. Ashfold and V. G. Stavros, J. Phys. Chem. Lett., 2015, 6, 1363–1368 CrossRef CAS PubMed.
- T. N. V. Karsili, B. Marchetti, M. N. R. Ashfold and W. Domcke, J. Phys. Chem. A, 2014, 118, 11999–12010 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc02381a |
| This journal is © The Royal Society of Chemistry 2016 |