Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoinduced electron transfer in porous organic salt crystals impregnated with fullerenes†
Tetsuya
Hasegawa
a,
Kei
Ohkubo
bc,
Ichiro
Hisaki
a,
Mikiji
Miyata
ad,
Norimitsu
Tohnai
*a and
Shunichi
Fukuzumi
*ce
aDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: tohnai@mls.eng.osaka-u.ac.jp
bDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
cDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Korea. E-mail: fukuzumi@chem.eng.osaka-u.ac.jp
dThe Institute of Scientific and Industrial Research, Osaka University, Ibaraki, Osaka 567-0047, Japan
eFaculty of Science and Technology, Meijo University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Nagoya, Aichi 468-8502, Japan
First published on 9th May 2016
Abstract
Porous organic salt (POS) crystals composed of 9-(4-sulfophenyl)anthracene (SPA) and triphenylmethylamine (TPMA) were impregnated with fullerenes (C60 and C70), which were arranged in one dimensional close contact. POS crystals of SPA and TPMA without fullerenes exhibit blue fluorescence due to SPA, whereas the fluorescence was quenched in POS with fullerenes due to electron transfer from the singlet excited state of SPA to fullerenes.
Metal–organic frameworks (MOFs) have attracted increasing attention as an interesting platform to design and develop artificial photosynthetic systems, because MOFs can contain light-harvesting and charge-separation units as well as catalytic units in a single crystal, providing the structural organization to integrate these units of artificial photosynthesis into a single crystal.1 There are many examples of MOFs in which energy and electron transfer has been investigated by fixing the distance and orientation between chromophores, which were precisely determined by single crystal X-ray crystallography.2–10
As compared with MOFs, porous organic salts (POSs) have advantage in terms of synthetic versatility, easier processing, more flexibility and better workability.11–15 In particular, POSs composed of ammonium cations and sulfonate anions have enabled various systematic designs of the resultant porous structures by changing the combination of ionic components using strong intermolecular interactions such as hydrogen bonds and electrostatic interactions.16,17 However, there has been no report on photoinduced electron transfer in POS crystals.
We report herein the first example of efficient photoinduced electron transfer in POS crystals composed of 9-(4-sulfophenyl)anthracene (SPA) and triphenylmethylamine (TPMA) impregnated with fullerenes (C60 and C70) as revealed by femtosecond laser flash photolysis and electronic paramagnetic resonance (EPR) measurements. Fullerenes were chosen as electron acceptors, because they are known to undergo efficient electron-transfer reduction with small reorganization energies.18 The combination of SPA and TPMA was chosen in order to make enough space for accommodation of fullerenes in the porous structure.
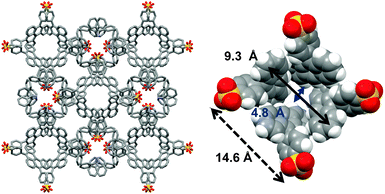
SPA was synthesized according to the literature (see the ESI†).19 The organic salt composed of SPA and TPMA yielded a pale yellow needle crystal of SPA/TPMA suitable for X-ray crystallographic analysis from an o-dichlorobenzene solution. The X-ray crystal structure of SPA/TPMA in Fig. 1 revealed that the crystal had a porous structure, which was hierarchically constructed (Fig. S1 in the ESI†). Four SPA molecules and four TPMA molecules assembled into a tetrahedral supramolecular cluster via a cubic-like charge-assisted hydrogen bond.20 Anthracene moieties of SPA were jutting out in the tetrahedral direction and located around the core structure. The tetrahedral clusters accumulated along the c-axis by CH–π interactions between anthracene moieties and trityl groups to form a linear column structure differently from the previously formed diamondoid structures.21 The column structures were bundled by CH–π interactions between anthracene moieties to give a bowl-like void space among the columns. The void spaces were connected leading to a channel type porous structure having a bottleneck (Fig. S2 in the ESI†). Recrystallization solvents were incorporated there but disordered.
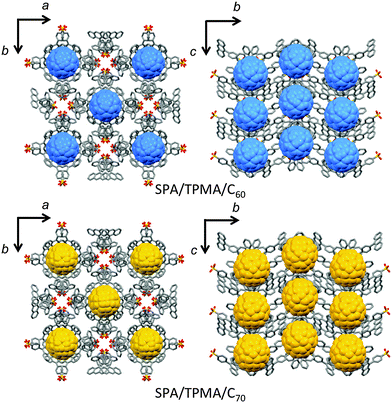
The void space of SPA/TPMA from o-dichlorobenzene with a diameter of 9.3 Å (Fig. 1) is capable of accommodating fullerenes. Recrystallization of SPA/TPMA with C60 and C70 gave purple-red and red-brown needle crystals, respectively. X-ray studies revealed that the porous organic salts composed of SPA and TPMA were impregnated with fullerenes as shown in Fig. 2. The crystallographic parameters of SPA/TPMA, SPA/TPMA/C60 and SPA/TPMA/C70 are shown in Table S1 (ESI†). The void space is enlarged by impregnation of fullerenes to be 10.8 Å and 11.1 Å in SPA/TPMA/C60 and SPA/TPMA/C70, respectively (Fig. S3 in the ESI†), because of comparatively weak interaction among the columns. This indicates the flexibility of the POS crystals. Moreover, the bottleneck in SPA/TPMA/C60 and SPA/TPMA/C70 is also expanded to be 6.6 Å and 7.2 Å, respectively. Consequently, the distance between fullerenes gets closer in the porous structures.
The SPA/TPMA crystal exhibits fluorescence due to the SPA moiety under photoirradiation (Fig. 3).22 In contrast, SPA/TPMA/C60 and SPA/TPMA/C70 exhibited no fluorescence probably because of electron transfer from the singlet excited state of SPA to the fullerene. The occurrence of the photoinduced electron transfer in SPA/TPMA/C60 crystals was confirmed by femtosecond laser flash photolysis measurements. The observed transient absorption spectra are shown in Fig. 4, where the transient absorption band at 580 nm of SPA (1SPA*) was observed upon femtosecond laser excitation and the decay is accompanied by an increase in the new absorption band at 700 nm due to the radical cation of SPA (SPA˙+).23 The concomitant formation of C60˙− was also observed at 1030 nm.18 Thus, photoinduced electron transfer from 1SPA* to C60 occurred in SPA/TPMA/C60 to produce the charge-separated (CS) state (SPA˙+/TPMA/C60˙−).
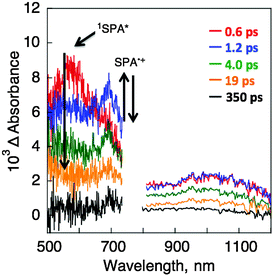 | ||
| Fig. 4 Transient absorption spectra of SPA/TPMA/C60 dispersed in the KBr pellet recorded at 0.6, 1.2, 4.0, 19 and 350 ps after femtosecond laser excitation at 393 nm. | ||
The rate constant of photoinduced electron transfer from 1SPA* to C60 was determined by the fast decay of absorbance at 600 nm due to 1SPA* to be 7.3 × 1011 s−1 (Fig. 5a). The decay time profile of absorbance at 700 nm due to SPA˙+ exhibited two components as shown in Fig. 5b, where the fast component afforded a rate constant of 7.3 × 1011 s−1, which agrees with the rate constant of electron transfer from 1SPA* to C60, and the slower component afforded a rate constant of 1.9 × 1010 s−1. This value is larger than the decay rate constant of C60˙− (5.7 × 109 s−1) determined by the slower decay of absorbance at 1030 nm (Fig. 5c). The faster decay of SPA˙+ than the decay of C60˙− indicates that electron transfer from TPMA to SPA˙+ occurred to produce the CS state (SPA/TPMA˙+/C60˙−), which decayed with a rate constant of 5.7 × 109 s−1 and a CS lifetime of 180 ps. Electron transfer from TPMA to SPA˙+ is indeed energetically feasible, because the one-electron oxidation potential of TPMA (1.20 V vs. SCE) is lower than that of SPA (1.40 V vs. SCE) as shown in their cyclic voltammograms (Fig. S5 in the ESI†).
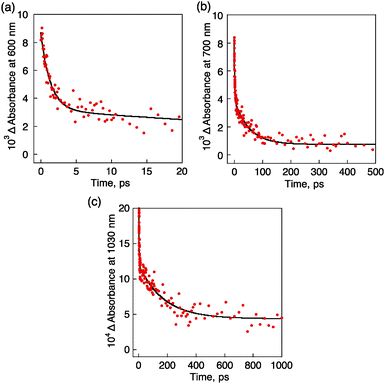 | ||
| Fig. 5 Decay time profiles of absorption at (a) 600 nm, (b) 700 nm and (c) 1030 nm of SPA/TPMA/C60 in the KBr pellet. | ||
Similarly a transient absorption spectrum of the CS state of SPA/TPMA/C70 was observed by femtosecond laser flash photolysis measurements (Fig. S6 in the ESI†). The rate constant of photoinduced electron transfer from 1SPA* to C70 was determined by the fast decay of absorbance at 600 nm due to 1SPA* to be 2.6 × 1011 s−1. The CS lifetime of SPA/TPMA˙+/C70˙− was also determined by the slower decay of absorbance at 1030 nm due to C70˙− to be 4.4 × 109 s−1, which corresponds to the lifetime of 230 ps.
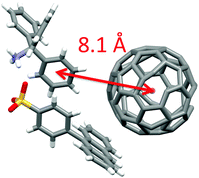
Upon photoirradiation (λ > 340 nm) of single crystals of SPA/TPMA/C60 in an ESR cavity at 77 K, we could observe a signal at g = 2.0025 as shown in Fig. 6. This signal can be assigned to the superposition of TPMA˙+ and C60˙−. In addition, we could observe a signal due to a triplet CS state, for which the zero-field splitting (D) value was determined to be 49 G. On the basis of this value, the distance between TPMA˙+ and C60˙− is estimated to be 8.3 Å,24 which is in good agreement with that found in the crystal structure (8.1 Å in Fig. 7).
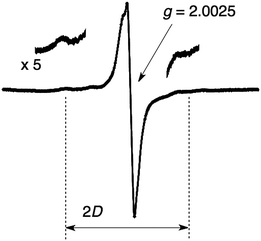 | ||
| Fig. 6 ESR spectrum recorded upon photoirradiation (λ > 340 nm) of crystals of SPA/TPMA/C60 at 77 K. | ||
In conclusion, porous organic salt (POS) crystals composed of 9-(4-sulfophenyl)anthracene (SPA) and triphenylmethylamine (TPMA) were successfully impregnated with fullerenes (C60 and C70) to reveal the X-ray crystal structures. Photoexcitation of POSs impregnated fullerenes resulted in efficient electron transfer from 1SPA* to fullerenes, followed by hole transfer from SPA˙+ to TPMA to produce the final CS states (SPA/TPMA˙+/C60˙− and SPA/TPMA˙+/C70˙−), which decayed with lifetimes of 180 and 230 ps, respectively. This work has demonstrated the excellent ability of POSs impregnated with fullerenes for efficient photoinduced electron transfer. The tunability and crystalline structures of POSs impregnated with various electron acceptors provide a promising platform to develop artificial photosynthetic systems.
This work was supported by Grants-in-Aid (No. 16H02268 to S. F., No. 25288036 to N. T., and No. 16K13964, 26620154 and 26288037 to K. O.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); ALCA and SENTAN projects to S. F. from JST, Japan.
Notes and references
- (a) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982 RSC; (b) M. C. So, G. P. Wiederrecht, J. E. Mondloch, J. T. Hupp and O. K. Farha, Chem. Commun., 2015, 51, 3501 RSC; (c) S. Wang and X. Wang, Small, 2015, 11, 3097 CrossRef CAS PubMed.
- K. Leong, M. E. Foster, B. M. Wong, E. D. Spoerke, D. Van Gough, J. C. Deaton and M. D. Allendorf, J. Mater. Chem. A, 2014, 2, 3389 CAS.
- S. Pullen, H. Fei, A. Orthaber, S. M. Cohen and S. Ott, J. Am. Chem. Soc., 2013, 135, 16997 CrossRef CAS PubMed.
- (a) C. A. Kent, D. Liu, T. J. Meyer and W. Lin, J. Am. Chem. Soc., 2012, 134, 3991 CrossRef CAS PubMed; (b) J.-L. Wang, C. Wang and W. Lin, ACS Catal., 2012, 2, 2630 CrossRef CAS.
- (a) W. A. Maza and A. J. Morris, J. Phys. Chem. C, 2014, 118, 8803 CrossRef CAS; (b) R. W. Larsen and L. Wojtas, J. Mater. Chem. A, 2013, 1, 14133 RSC.
- K. G. M. Laurier, E. Fron, P. Atienzar, K. Kennes, H. Garcia, M. Van der Auweraer, D. E. De Vos, J. Hofkens and M. M. J. Roeffaers, Phys. Chem. Chem. Phys., 2014, 16, 5044 RSC.
- B. Ferrer, M. Alvaro, H. G. Baldovi, H. Reinsch and N. Stock, ChemPhysChem, 2014, 15, 924 CrossRef CAS PubMed.
- Y. Zeng, Z. Fu, H. Chen, C. Liu, S. Liao and J. Dai, Chem. Commun., 2012, 48, 8114 RSC.
- L. Han, L. Qin, L.-P. Xu and W.-N. Zhao, Inorg. Chem., 2013, 52, 1667 CrossRef CAS PubMed.
- M. Alvaro, E. Carbonell, B. Ferrer, F. X. Llabrés i Xamena and H. Garcia, Chem. – Eur. J., 2007, 13, 5106 CrossRef CAS PubMed.
- H. Nobukuni, F. Tani, Y. Shimazaki, Y. Naruta, K. Ohkubo, T. Nakanishi, T. Kojima, S. Fukuzumi and S. Seki, J. Phys. Chem. C, 2009, 113, 19694 CAS.
- A. I. Cooper, Angew. Chem., Int. Ed., 2012, 51, 7892 CrossRef CAS PubMed.
- (a) P. Li, Y. He, J. Guang, L. Weng, J. C.-G. Zhao, S. Xiang and B. Chen, J. Am. Chem. Soc., 2014, 136, 547 CrossRef CAS PubMed; (b) Y. He, S. Xiang and B. Chen, J. Am. Chem. Soc., 2011, 133, 14570 CrossRef CAS PubMed.
- (a) I. Oueslati, J. A. Paixâo, V. H. Rodrigues, K. Suwinska, B. Lesniewska, A. Shkurenko, M. E. S. Eusébio, J. Vicens, T. M. R. Maria and A. L. Ramalho, Cryst. Growth Des., 2013, 13, 4512 CrossRef CAS; (b) Y.-N. Li, L.-H. Huo, Z.-P. Deng, X. Zou, Z.-B. Zhu, H. Zhao and S. Gao, Cryst. Growth Des., 2014, 14, 2381 CrossRef CAS.
- (a) A. Comotti, S. Bracco, A. Yamamoto, M. Beretta, T. Hirukawa, N. Tohnai, M. Miyata and P. Sozzani, J. Am. Chem. Soc., 2014, 136, 618 CrossRef CAS PubMed; (b) M. Sugino, K. Hatanaka, Y. Araki, I. Hisaki, M. Miyata and N. Tohnai, Chem. – Eur. J., 2014, 20, 3069 CrossRef CAS PubMed; (c) M. Sugino, K. Hatanaka, T. Miyano, I. Hisaki, M. Miyata, A. Sakon, H. Uekusa and N. Tohnai, Tetrahedron Lett., 2014, 55, 732 CrossRef CAS.
- (a) Y. Mizobe, M. Miyata, I. Hisaki, Y. Hasegawa and N. Tohnai, Org. Lett., 2006, 8, 4295 CrossRef CAS PubMed; (b) T. Hinoue, M. Miyata, I. Hisaki and N. Tohnai, Angew. Chem., Int. Ed., 2012, 51, 155 CrossRef CAS PubMed.
- (a) M. Sugino, Y. Araki, K. Hatanaka, I. Hisaki, M. Miyata and N. Tohnai, Cryst. Growth Des., 2013, 13, 4986 CrossRef CAS; (b) A. Yamamoto, T. Hasegawa, T. Hamada, T. Hirukawa, I. Hisaki, M. Miyata and N. Tohnai, Chem. – Eur. J., 2013, 19, 3006 CrossRef CAS PubMed.
- (a) S. Fukuzumi, K. Ohkubo, H. Imahori and D. M. Guldi, Chem. – Eur. J., 2003, 9, 1585 CrossRef CAS PubMed; (b) S. Fukuzumi, T. Suenobu, M. Patz, T. Hirasaka, S. Itoh, M. Fujitsuka and O. Ito, J. Am. Chem. Soc., 1998, 120, 8060 CrossRef CAS; (c) Y. Kawashima, K. Ohkubo and S. Fukuzumi, J. Phys. Chem. A, 2013, 117, 6737 CrossRef CAS PubMed.
- (a) A. Etienne and C. Degent, FR. Pat., 1085860, 1955 Search PubMed; (b) D. P. Getman, G. A. Decrescenzo, J. N. Freskos, M. L. Vazquez, J. A. Sikorski, B. Devadas, S. R. Nagarajan, D. L. Brown and J. J. McDonald, US Pat., 6388132, 2000 Search PubMed.
- (a) N. Tohnai, Y. Mizobe, M. Doi, S. Sukata, T. Hinoue, T. Yuge, I. Hisaki, Y. Matsukawa and M. Miyata, Angew. Chem., Int. Ed., 2007, 46, 2220 CrossRef CAS PubMed; (b) M. Miyata, N. Tohnai and I. Hisaki, Acc. Chem. Res., 2007, 40, 694 CrossRef CAS PubMed.
- (a) A. Yamamoto, S. Uehara, T. Hamada, M. Miyata, I. Hisaki and N. Tohnai, Cryst. Growth Des., 2012, 12, 4600 CrossRef CAS; (b) A. Yamamoto, T. Hamada, I. Hisaki, M. Miyata and N. Tohnai, Angew. Chem., Int. Ed., 2013, 52, 1709 CrossRef CAS PubMed; (c) A. Yamamoto, T. Hirukawa, I. Hisaki, M. Miyata and N. Tohnai, Tetrahedron Lett., 2013, 54, 1268 CrossRef CAS.
- UV-vis and fluorescence spectra of SPA/TPMA in PhCN are shown in Fig. S4 in the ESI†.
- S. Fukuzumi, I. Nakanishi and K. Tanaka, J. Phys. Chem. A, 1999, 103, 11212 CrossRef CAS.
- The distance (r, Å) between the two radical centres was estimated using the following equation: r = [(2.78 × 104)/D]1/3.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and spectral data. CCDC 1044710–1044712. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc02377k |
| This journal is © The Royal Society of Chemistry 2016 |