 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A fishing rod-like conjugated polymer bearing pillar[5]arenes†
Yingjie
Ma
a,
Long
Chen
b,
Chen
Li
a and
Klaus
Müllen
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: muellen@mpip-mainz.mpg.de; Fax: +49 6131-379-350
bDepartment of Chemistry, School of Science, Tianjin University, 300072 Tianjin, People's Republic of China
First published on 4th April 2016
Abstract
A novel diethynyl functionalized pillar[5]arene, that is a pentabenzenacyclodecaphane derivative, has been synthesized and polymerized with p-dihalobenzene to form a fishing rod-like conjugated polymer, where one of the benzenes in the pillar[5]arene rings is incorporated into a poly(arylene ethynylene) backbone.
Chemists can tailor conjugated polymers (CPs) for various physical and electronic properties by introducing functional groups,1–5 such as macrocycles either as part of the polymer backbone or as substituents. Via integration of different kinds of macrocycles into conjugated polymer backbones, the polymers could be endowed with novel roles merging chemoreceptivity, electronics and supramolecular chemistry. Fig. 1 illustrates two modes for their incorporation into the polymer backbone. Swager and co-workers inserted calix[4]arene (at the upper rim) into thiophene-based polymers, such that the macrocycle forms a bridge, and obtained an electroactive zigzag structure (Fig. 1a).6a Alternately, Vigalok et al. reported a polymer incorporating only a single benzene ring of each tetrabenzenacyclodecaphane rings into the backbone, giving fluorescent materials that could detect gaseous nitric oxide via reversible complexation (Fig. 1b).6b
Pillararenes, especially pillar[5]arenes (compounds 2–4, Scheme 1), a new class of macrocycles, i.e. pentabenzenacyclodecaphanes, have attracted much interest recently,7a due to their facile synthesis as well as versatile nature as chemoreceptors. Different from the meta-bridged calixarenes, pillararene rings are formed by methylene bridges through para-phenylenes. Pillar[5]arenes are good host molecules for different electron-accepting guests or other neutral molecules, for instance dialkyl viologens,7a alkylated pyridiniums7f and n-hexane.8a Moreover, they can be easily functionalized especially on the benzene rings, rendering them to be promising vesicles,8e artificial transmembrane channels,9b nanotubes,8d nanoparticles,8f and other supramolecular systems for chemical and biological applications.7–10
Up to now there have been only a few examples of pillar[5]arene incorporated CPs, such as pillar[5]arenes as tethered side chain functionalized polyphenylethynylene,9c and the Zylon poly(p-phenylene-2,6-benzobisoxazole) derivative generated by the polymerization of one benzene unit in the pillar[5]arene monomer.7g Herein, we synthesized a novel bifunctional pillar[5]arene monomer bearing two alkynyl groups, from which a new pillar[5]arene-based conjugated polymer (1) was prepared by the Sonogashira polycondensation, as illustrated in Scheme 1.
Compound 4 with two hydroxyl groups was treated with trifluoromethanesulfonic anhydride to give ditriflated pillar[5]arene (compound 3).7b Subsequently, the Pd-catalyzed Suzuki–Miyaura cross-coupling of 3 with trimethyl((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethynyl)silane 6 followed by removing the trimethylsilyl groups led to the targeted pillar[5]arene 2 containing two ethynyl groups for polymerization. The structure of 2 was confirmed by 1H, 13C, 2D NMR and high resolution mass spectrometry (Fig. S2–S7, ESI†).
Single crystals of 2 were obtained by the diffusion of MeOH into a dichloromethane solution of 2. The X-ray crystallographic analysis indicates the presence of enantiomers in the unit cell (Fig. 2). The formation of pS and pR enantiomers is due to the molecule's planar chirality—the phenylene units of the parent dimethoxypillar[5]arene can rotate around the methylene bridges, affording two conformations (pS and pR), which however easily interconvert in solution. Nevertheless, the bulky 4-ethynylphenyl groups in 2 prevent the rotation of the units and thus inhibit the interconversion between the two conformations. As a consequence, the pS and pR enantiomers are stabilized.7d It is also noticeable that a dichloromethane molecule is encapsulated in the cavity of each molecule of compound 2. Particularly, the distances between the hydrogen atoms of the dichloromethane molecule and the corresponding phenyl ring of pillar[5]arenes (the bonds A, B, A′ and B′ in Fig. 2) is only 2.71 Å, which indicates the C–H·π interactions between dichloromethane and the cavity of 2 (see the blue dotted lines in Fig. 2). This is similar to the case of mono-octyl-substituted copillar[5]arene in the solid state.8a
In order to polymerize 2 under Sonogashira conditions, we prepared monomer 5 as the dihalo-aromatic comonomer (Fig. S8, ESI†). After combining 2 and 5 with a catalytic amount of Pd(PPh3)4 and CuI in diisopropylamine–toluene, the crude polymer could be easily purified by precipitation from dichloromethane by addition of methanol to give the target polymer 1 as a yellow solid (94% isolated yield).
According to gel permeation chromatography (GPC), the relative number-average molecular weight (Mn) of CP1 was about 16 kDa with a polydispersity index (PDI) of 3.82 (THF eluent, polystyrene standards, Fig. S10, ESI†). This corresponds to an average of thirteen repeat units in the polymer backbone. Polymer 1 is highly soluble in common organic solvents (CHCl3, CH2Cl2, THF, and toluene) allowing thorough characterization by 1H NMR spectroscopy. The 1H NMR spectrum of 1 indicated that proton signals assigned to pillar[5]arene benzene rings and methoxy groups shifted upfield and downfield, respectively, compared with those of monomer 2 (Fig. S9, ESI†). The polymer structure is further supported by the matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) spectrum, which exhibited a series of peaks, regularly separated by the molar mass of the repeat unit (1220, Fig. S11, ESI†). All the results above confirmed the successful synthesis of 1 bearing pillar[5]arenes in the conjugated backbone. Polymer 1 can be viewed as a fishing rod-like conjugated “hydrocarbon nanotube”6b – the conjugated backbone is the rod and the pillar[5]arene units are the guides arranged along the underside of the fishing rod (Scheme 1).
The UV-visible absorption and fluorescence spectra of polymer 1 were compared to those of monomer 2 (Fig. 3). The extended conjugation had a profound effect on the UV-visible and fluorescence properties of the pillar[5]arene compound (Table 1)—monomer 2 has absorption maximum at 284 nm, while 1 has absorption maxima at 306 and 378 nm with a significant bathochromic shift of 94 nm. On the other hand, monomer 2 is hardly fluorescent, but 1 is highly blue fluorescent with emission maximum at 416 nm, as a result of the formation of the conjugated backbone.
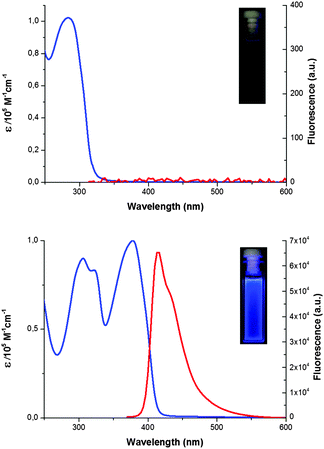 | ||
| Fig. 3 UV-visible absorption (blue line) and fluorescence (red line) spectra of monomers 2 (top, 10−5 M) and 1 (bottom, [RU] = 10−5 M, chloroform). | ||
| M n (kDa) | PDI | Solution (CHCl3) | Film | |||
|---|---|---|---|---|---|---|
| Abs λmax (nm) | Em λmax (nm) | Abs λmax (nm) | Em λmax (nm) | |||
| 1 | 16 | 3.8 | 306, 378 | 416 | 303, 377 | 429 |
Compared to the adsorption spectrum in solution, the broader adsorption spectrum of 1 in the film indicates that aggregation occurs in the solid state (Fig. 4). Furthermore, the emission peak of 1 shows a noticeable red-shift (∼13 nm) in the solid state relative to the solution spectrum.
 | ||
| Fig. 4 Normalized absorption and emission spectra of 1 in CHCl3 (red/solid) and in thin film (blue/dashed). | ||
In summary, we have described an efficient pathway towards a new bifunctional pillar[5]arene monomer bearing two alkynyl groups for easy and efficient polymerization under Sonogashira conditions to achieve a fishing rod-like conjugated polymer. The new polymer has the features of both semiconductor polymers and supramolecular structures. Further investigation of electronic sensing devices is underway in our laboratory to provide application information of polymer 1.
This work was supported by the VW foundation (AZ. 85101-85103), SENSOR.
Notes and references
- (a) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed; (b) F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS PubMed; (c) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef PubMed; (d) J. Pecher and S. Mecking, Chem. Rev., 2010, 110, 6260 CrossRef CAS PubMed; (e) S. Rochat and T. M. Swager, J. Am. Chem. Soc., 2013, 135, 17703 CrossRef CAS PubMed.
- A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897 CrossRef CAS PubMed.
- (a) A. R. Murphy and J. M. J. Fréchet, Chem. Rev., 2007, 107, 1066 CrossRef CAS PubMed; (b) C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed.
- (a) S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef PubMed; (b) J. Peet, A. J. Heeger and G. C. Bazan, Acc. Chem. Res., 2009, 42, 1700 CrossRef CAS PubMed; (c) P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed.
- (a) X. Feng, L. Liu, S. Wang and D. Zhu, Chem. Soc. Rev., 2010, 39, 2411 RSC; (b) X. Ji, Y. Yao, J. Li, X. Yan and F. Huang, J. Am. Chem. Soc., 2013, 135, 74 CrossRef CAS PubMed.
- (a) H. Yu, B. Xu and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 11142 CrossRef PubMed; (b) A. Molad, I. Goldberg and A. Vigalok, J. Am. Chem. Soc., 2012, 134, 7290 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) N. L. Strutt, D. Fairen-Jimenez, J. Iehl, M. B. Lalonde, R. Q. Snurr, O. K. Farha, J. T. Hupp and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 17436 CrossRef CAS PubMed; (c) N. L. Strutt, H. Zhang, M. A. Giesener, J. Lei and J. F. Stoddart, Chem. Commun., 2012, 48, 1647 RSC; (d) T. Ogoshi, D. Yamafuji, T. Akutsu, M. Naito and T. Yamagishi, Chem. Commun., 2013, 49, 8782 RSC; (e) S. Kanai, Y. Nojiri, G. Konishi and Y. Nakamoto, Polym. Prep. Jpn. J., 2006, 55, 303 Search PubMed; (f) C. Li, Q. Xu, J. Li, Y. Feina and X. Jia, Org. Biomol. Chem., 2010, 8, 1568 RSC; (g) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Chem. – Eur. J., 2014, 20, 10996 CrossRef CAS PubMed.
- (a) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Org. Lett., 2010, 12, 3285 CrossRef CAS PubMed; (b) C. Han, F. Ma, Z. Zhang, B. Xia, Y. Yu and F. Huang, Org. Lett., 2010, 12, 4360 CrossRef CAS PubMed; (c) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed; (d) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712 CrossRef CAS PubMed; (e) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248 CrossRef CAS PubMed; (f) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed.
- (a) X.-B. Hu, L. Chen, W. Si, Y. Yu and J.-L. Hou, Chem. Commun., 2011, 47, 4694 RSC; (b) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564 CrossRef CAS PubMed; (c) S. Sun, X. Hu, D. Chen, J. Shi, Y. Dong, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 2224 RSC.
- (a) T. Adiri, D. Marcian and Y. Cohen, Chem. Commun., 2013, 49, 7082 RSC; (b) Y. Yang, Y. Sun and N. Song, Acc. Chem. Res., 2014, 47, 1950 CrossRef CAS PubMed; (c) Z. Li, Y. Zhang, C. Zhang, L. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization. CCDC 1456830. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc02059c |
| This journal is © The Royal Society of Chemistry 2016 |



