Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
UV-induced epitaxial attachment of TiO2 nanocrystals in molecularly mediated 1D and 2D alignments
Tomoyuki
Hiraide
,
Hiroyuki
Kageyama
,
Yoshitaka
Nakagawa
,
Yuya
Oaki
and
Hiroaki
Imai
*
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1, Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: hiroaki@applc.keio.ac.jp
First published on 29th April 2016
Abstract
Anatase TiO2 nanocrystals were crystallographically connected through UV-induced epitaxial attachments in 1D and 2D arrays with photocatalytic decomposition of the organic mediator.
The bottom-up approach is an important route to nanometric materials that have great advantages over the bulky structures obtained using top-down processes.1,2 Oriented attachment is a prospective non-classical bottom-up process for constructing a large-scale assembly of nanocrystals, such as biominerals.2,3 One-, two-, and three-dimensional (1D, 2D, and 3D) crystals were found to be grown through the oriented attachment of nanoscale building blocks.4
Generally, superlattice structures are formed through the self-assembly of mono, binary, and ternary compounds of spherical nanocrystals.5 Especially, the crystallographic orientation of superlattices is ordered by cubic-shaped nanocrystals.6 Anisotropic shaped nanocrystals have the potential to form a molecular-mediated structure with a specific alignment.7 1D, 2D, and 3D microarrays of rectangular Mn3O4 nanoblocks were produced with controllable a and c axis orientations.7c In our previous report, 1D chains that consisted of crystallographically continuous Mn3O4 nanocuboids were achieved via the removal of organic mediators by plasma treatment and heat treatment.8 Thus, such anisotropic nanoblocks have great potential in the construction of designed crystal morphologies.
Today, TiO2 is used for various applications, such as photocatalysts, solar cell electrodes, and anodes of lithium-ion batteries.4a,9–11 The shape and crystal surfaces of anatase TiO2 nanocrystals were found to influence their photocatalytic activity.10 Bulk heterojunction solar cells were fabricated with TiO2 nanorods, polymers, and dyes.11 Recently, the shape and crystal surfaces of TiO2 nanoparticles have been finely controlled using organic molecules, such as oleic acid and oleylamine.12 Moreover, the horizontal and vertical 2D superlattices were produced with anisotropic TiO2 nanocrystals.13 However, the selective assembly of 1D and 2D arrays has not yet been achieved using nanocrystals. The purpose of our present study is to examine selective 1D and 2D alignments of TiO2 nanocrystals and their epitaxial attachments under UV irradiation. Single-crystalline TiO2 alignments would be obtained through crystallographic fusion via the removal of organic molecules using a non-thermal process.
Dispersion of TiO2 nanocrystals was induced using a solvothermal method based on previous studies.12a,13 Five mmol of titanium butoxide (TB), 10 mmol of oleic acid (OA), and 15 mmol of oleylamine (OM) were mixed and stirred in 2.7 cm3 of dehydrated ethanol. The mixture, in a Teflon vessel, was put into a larger Teflon vessel, and a 10 cm3 mixture of dehydrated ethanol and pure water (ethanol/water = 24 in volume) was poured around the smaller vessel. The solvothermal process was carried out at 180 °C for ∼18 h in a stainless steel autoclave. The precipitate at the bottom of the dispersion was extracted, and then toluene, OA, and OM were added. The precipitate obtained by the centrifugation of the toluene dispersion was redispersed in hexane. A transmission electron microscope (TEM, FEI Tecnai G2 F20) operated at 120–200 kV was used to observe the nanostructures of the products. The organic compounds in the obtained powders were analyzed using the KBr method using Fourier transform infrared spectroscopy (FTIR, Jasco FT/IR-4200). UV light from a super high-pressure UV lamp (Ushio UPM2-252Q) through visible and near infrared filters was irradiated onto the nanocrystals for the decomposition of the organic molecules.
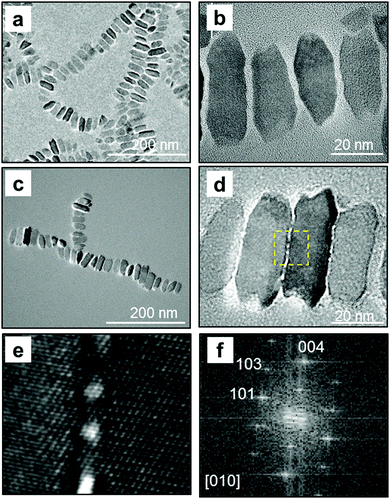
Anatase TiO2 nanocrystals were obtained as a dispersion in toluene. The crystalline phase was confirmed by lattice fringes observed using high-resolution (HR) TEM (Fig. 1). The nanocrystal was elongated along the c axis of anatase, which has a tetragonal structure. The nanocrystal had a truncated rectangular shape, and the lengths of the short a axis and the long c axis were ∼16 and ∼34 nm, respectively. According to the previous study,12 OA and OM adsorb to the {001} and {101} faces of anatase and control the growth rate in the [001] and [101] directions, respectively. Moreover, the growth rate of TiO2 crystals influences the shape of the nanoparticles.12a In the present study, the {100} facets of anatase nanocrystals are deduced to be induced by the combined effects of TB, OA, and OM. These anisotropic nanocrystals are appropriate for the assembly of 1D and 2D patterns.
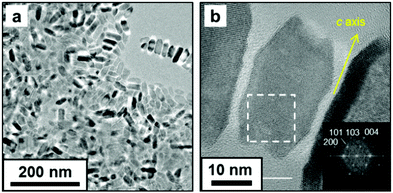 | ||
| Fig. 1 TEM (a) and HRTEM (b) images of obtained nanocrystals in toluene. The fast Fourier transform (FFT) profile in the dashed square is shown in inset (b). | ||
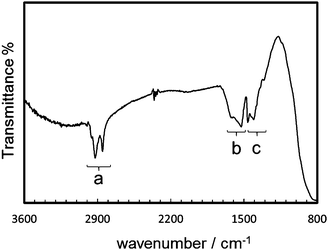
The extraction and redispersion of the resultant nanocrystals in hexane were necessary for separating nanocrystals with similar shapes and sizes. Screened nanocrystals were found to form molecular-mediated alignment, as shown in TEM images (Fig. 2a and b). The ordered 1D alignment occurred in the a direction. The formation mechanism of the specifically ordered arrays was reported in our previous report.7c The nanoblocks are arranged at the liquid–air interface due to their hydrophobic nature. The 1D chains elongated in the 〈100〉 direction are formed at the interface through the attachment of the relatively large (100) faces by lateral capillary force when the medium is evaporated. The lengths of interspace between two nanocrystals were 1.7–3.0 nm, which indicated that the organic molecules exist in the interspace. The FTIR spectrum (Fig. 3) indicated the presence of OA and OM on the nanocrystals. The bands in the region (a), 2950, 2920, and 2850 cm−1, are assigned to the antisymmetric stretching vibration of –CH3– and the antisymmetric and symmetric stretching vibrations of –CH2– groups, respectively. The bands in the region (b), 1620–1525 cm−1, were assigned to the scissoring bending vibration of groups N–H, and the antisymmetric and symmetric stretching vibrations of COO− groups. The bands in the region (c), 1460–1405 cm−1, were assigned to the bending vibrations of C–O–H, –CH3–, and O–H groups.6a,14 The organic molecules mediated the specific alignment of the nanocrystals.
Fig. 2c–f shows TEM images of TiO2 nanocrystals on a nitrocellulose-coated TEM grid after ∼18 h of UV irradiation. The photocatalytic effect decomposed the organic molecules adsorbed on the nanocrystal surface. Continuous lattice fringes through several crystal bridges were observed between the adjacent nanocrystals (Fig. 2e). These results indicate that epitaxial attachment occurred with UV irradiation. The organic molecules on the nanocrystal surface would shrink with decomposition, and two nanocrystals would approach each other. The approximation of nanocrystals with the same a direction caused crystallographic fusion without heat. As the organic molecules decomposed, the bare surfaces were crystallographically connected to each other.
In the previous study, 2D clusters with attachment on the a faces of Mn3O4 nanocuboids were formed with the increasing polarity of the dispersion medium.7cFig. 4a shows the TiO2 nanoblocks on a TEM grid from their dispersion in tetrahydrofuran (THF), which has a higher polarity than hexane. A number of square-shaped crystal arrays were observed on the TEM grid. This indicates that 2D arrays with c faces parallel to the substrate were obtained in a part of the nanoblocks. In a relatively polar medium, 2D arrays of the hydrophobic nanoblocks are easily formed via the attachment of the relatively large (100) faces. The 2D arrays were also obtained from dispersion in hexane in the presence of an additional amount of OA (Fig. 4b). We found the formation of 2D arrays with increasing concentrations of organic molecules. In the previous study, 2D superlattices with the c axis vertical to a substrate were obtained with increasing nanocrystals and OA.13 The excess amount of the surface-mediated molecules enhanced the interaction, such as depletion attraction,15 between TiO2 nanocrystals.
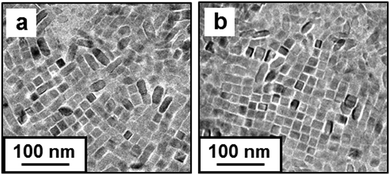 | ||
| Fig. 4 TEM images of the TiO2 nanocrystals on TEM grids from the dispersion in tetrahydrofuran (a), and in hexane with additional amounts of OA (b). | ||
Here, crystallographically connected 2D arrays were formed with UV irradiation. The 2D arrays of TiO2 nanocrystals, in which the c face was parallel to the substrate, were obtained on a TEM grid by dispersion in toluene with additional OA and OM. After UV irradiation, the nanocrystals were connected in the arrays (Fig. 5). With UV irradiation, the average distance of interspace was decreased from 2.7 ± 0.50 nm to 0.40 ± 0.51 nm. The decomposition of organic molecules and the epitaxial attachment progressed on four sides of a TiO2 nanocrystal in the 2D arrays.
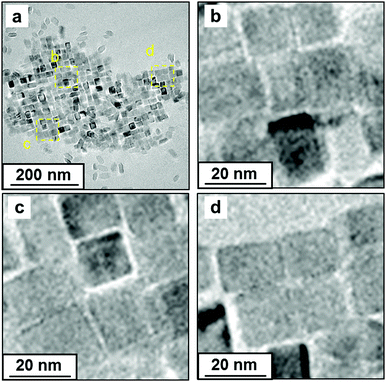 | ||
| Fig. 5 TEM images of the oriented TiO2 nanocrystals on a TEM grid after UV irradiation for 14 h (a–d). Images (b–d) show magnified images of the yellow-dashed square in (a). | ||
The 1D alignments of Mn3O4 nanoblocks,7c that had no photocatalytic activity, were irradiated for 18 h by UV light from the same super high-pressure UV lamp as a reference. The epitaxial attachment with the removal of organic molecules was not observed on the Mn3O4 nanoblocks, although UV light would be absorbed by the metal oxide. This suggests that the heat-induced phenomenon is not essential for the epitaxial attachment of TiO2 nanoblocks under UV irradiation. The organic molecules that were closely attached onto TiO2 nanoblocks were decomposed by UV irradiation. In this case, the organic molecules would be directly decomposed by the photocatalytic activation rather than the exothermal oxidative reaction with oxygen molecules. Thus, the oxidative pyrolysis is not the key reaction for the epitaxial attachment.
In summary, 1D and 2D alignments of anatase TiO2 were selectively achieved via evaporation-induced assembly. Epitaxial attachment of the nanocrystals in the ordered arrays was induced by the decomposition of organic molecules on nanocrystal surfaces and the approximation of nanocrystals under UV irradiation. Photocatalytic activity enables the crystallographic fusion of oriented nanoblocks through a non-thermal process.
Notes and references
- (a) C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater. Sci., 2000, 30, 545–610 CrossRef CAS; (b) K. Zhou and Y. Li, Angew. Chem., Int. Ed., 2012, 51, 602–613 CrossRef CAS PubMed.
- (a) H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576–5591 CrossRef PubMed; (b) Q. Zhang, S.-J. Liu and S.-H. Yu, J. Mater. Chem., 2009, 19, 191–207 RSC.
- (a) R. L. Penn and J. F. Banfield, Am. Mineral., 1998, 83, 1077–1082 CrossRef CAS; (b) C. Schliehe, B. H. Juarez, M. Pelletier, S. Jander, D. Greshnykh, M. Nagel, A. Meyer, S. Foerster, A. Kornowski, C. Klinke and H. Weller, Science, 2010, 329, 550–553 CrossRef CAS PubMed; (c) M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393–395 CrossRef CAS; (d) C. J. Dalmaschio, C. Ribeiro and E. R. Leite, Nanoscale, 2010, 2, 2336–2345 RSC; (e) Z. Zhuang, X. Xue and Z. Lin, Phys. Chem. Chem. Phys., 2015, 17, 4845–4848 RSC.
- (a) R. L. Penn and J. F. Banfield, Geochim. Cosmochim. Acta, 1999, 63, 1549–1557 CrossRef CAS; (b) M. Adachi, Y. Murata, J. Takao, J. Jiu, M. Sakamoto and F. Wang, J. Am. Chem. Soc., 2004, 126, 14943–14949 CrossRef CAS PubMed; (c) C.-S. Tsao, C.-M. Chuang, C.-Y. Chen, Y.-C. Huang, H.-C. Cha, F.-H. Hsu, C.-Y. Chen, Y.-C. Tu and W.-F. Su, J. Phys. Chem. C, 2014, 118, 26332–26340 CrossRef CAS; (d) H. Wang, Y. Liu, Z. Liu, H. Xu, Y. Deng and H. Shen, CrystEngComm, 2012, 14, 2278–2282 RSC.
- E. V. Shevchenko, D. V. Talapin, N. A. Kotov, S. O'Brien and C. B. Murray, Nature, 2006, 439, 55–59 CrossRef CAS PubMed.
- (a) S. Yang and L. Gao, J. Am. Chem. Soc., 2006, 128, 9330–9331 CrossRef CAS PubMed; (b) J. Ren and R. D. Tilley, J. Am. Chem. Soc., 2007, 129, 3287–3291 CrossRef CAS PubMed.
- (a) T. S. Sreeprasad, A. K. Samal and T. Pradeep, Langmuir, 2008, 24, 4589–4599 CrossRef CAS PubMed; (b) A. Singh, R. D. Gunning, S. Ahmed, C. A. Barrett, N. J. English, J.-A. Garate and K. M. Ryan, J. Mater. Chem., 2012, 22, 1562–1569 RSC; (c) Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, J. Am. Chem. Soc., 2014, 136, 3716–3719 CrossRef CAS PubMed.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, Langmuir, 2015, 31, 6197–6201 CrossRef CAS PubMed.
- (a) G. Liu, H. G. Yang, J. Pan, Y. Q. Yang, G. Q. Lu and H.-M. Cheng, Chem. Rev., 2014, 114, 9559–9612 CrossRef CAS PubMed; (b) L. Etgar, W. Zhang, S. Gabriel, S. G. Hickey, M. K. Nazeeruddin, A. Eychmüller, B. Liu and M. Grätzel, Adv. Mater., 2012, 24, 2202–2206 CrossRef CAS PubMed; (c) N. Li, G. Zhou, R. Fang, F. Li and H.-M. Cheng, Nanoscale, 2013, 5, 7780–7784 RSC.
- (a) R. Menzel, A. Duerrbeck, E. Liberti, H. C. Yau, D. McComb and M. S. P. Shaffer, Chem. Mater., 2013, 25, 2137–2145 CrossRef CAS; (b) T. R. Gordon, M. Cargnello, T. Paik, F. Mangolini, R. T. Weber, P. Fornasiero and C. B. Murray, J. Am. Chem. Soc., 2012, 134, 6751–6761 CrossRef CAS PubMed; (c) J. Pan, G. Liu, G. Q. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133–2137 CrossRef CAS PubMed; (d) X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- (a) T.-W. Zeng, Y.-Y. Lin, H.-H. Lo, C.-W. Chen, C.-H. Chen, S.-C. Liou, H.-Y. Huang and W.-F. Su, Nanotechnology, 2006, 17, 5387–5392 CrossRef CAS; (b) Y.-Y. Lin, T.-H. Chu, S.-S. Li, C.-H. Chuang, C.-H. Chang, W.-F. Su, C.-P. Chang, M.-W. Chu and C.-W. Chen, J. Am. Chem. Soc., 2009, 131, 3644–3649 CrossRef CAS PubMed; (c) Y.-C. Huang, G. C. Welch, G. C. Bazan, M. L. Chabinyc and W.-F. Su, Chem. Commun., 2012, 48, 7250–7252 RSC.
- (a) C.-T. Dinh, T.-D. Nguyen, F. Kleitz and T.-O. Do, ACS Nano, 2009, 3, 3737–3743 CrossRef CAS PubMed; (b) P. D. Cozzoli, A. Kornowski and H. Weller, J. Am. Chem. Soc., 2003, 125, 14539–14548 CrossRef CAS PubMed; (c) B. H. Wu, G. Y. Guo, N. F. Zheng, Z. X. Xie and G. D. Stucky, J. Am. Chem. Soc., 2008, 130, 17563–17567 CrossRef CAS PubMed.
- Y. Zhang and F.-M. Liu, RSC Adv., 2015, 5, 66934–66939 RSC.
- M. Kar, R. Agrawal and H. W. Hillhouse, J. Am. Chem. Soc., 2011, 133, 17239–17247 CrossRef CAS PubMed.
- D. Baranov, A. Fiore, M. V. Huis, C. Giannini, A. Falqui, U. Lafont, H. Zandbergen, M. Zanella, R. Cingolani and L. Manna, Nano Lett., 2010, 10, 743–749 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |