Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nickel(0)-catalyzed intramolecular reductive coupling of alkenes and aldehydes or ketones with hydrosilanes†
Yukari
Hayashi
a,
Yoichi
Hoshimoto
ab,
Ravindra
Kumar
a,
Masato
Ohashi
a and
Sensuke
Ogoshi
*a
aDepartment of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: ogoshi@chem.eng.osaka-u.ac.jp
bFrontier Research Base for Global Young Researchers, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
First published on 4th April 2016
Abstract
A nickel(0)-catalyzed reductive coupling of aldehydes and simple alkenes with hydrosilanes has been developed. A variety of silyl-protected 1-indanol derivatives were prepared in a highly diastereoselective manner (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) by employing a combination of nickel(0)/N-heterocyclic carbene and triethylsilane. The present system was also applied to a reductive coupling with ketones. Preliminary results of a nickel(0)-catalyzed asymmetric three-component coupling reaction of an aldehyde, an alkene, and triethylsilane are also shown.
1 dr) by employing a combination of nickel(0)/N-heterocyclic carbene and triethylsilane. The present system was also applied to a reductive coupling with ketones. Preliminary results of a nickel(0)-catalyzed asymmetric three-component coupling reaction of an aldehyde, an alkene, and triethylsilane are also shown.
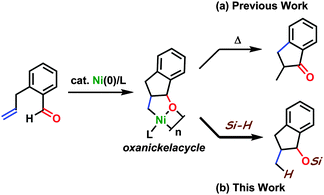
A high level of efficiency and wide applicability would make the oxidative cyclization of two π components with nickel(0) a key process in a variety of catalytic molecular transformations proceeding through carbon–carbon bond formation. Indeed, much progress has been made in nickel(0)-catalyzed molecular transformations, which were proposed to take place through hetero-nickelacycle intermediates generated by oxidative cyclization.1,2 We have also contributed to these developments1a,b by the direct observation of the formation of oxanickelacycles from (η2-aldehyde:η2-alkene)nickel complexes through oxidative cyclization,3a and by the expansion of this stoichiometric process to a catalytic hydroacylation yielding five- and six-membered benzocyclic ketones (Scheme 1a).3b This hydroacylation was proposed to proceed through β-hydride elimination from the oxanickelacycle intermediate, which was supported by the results of stoichiometric experiments. Oxanickelacycles could efficiently react with a reductant such as silanes, organoboranes, and organozincs, and these have been the key for many nickel(0)-catalyzed reductive coupling reactions.2 Among them, the reactions of alkynes and aldehydes have been well-developed.4,5 The use of 1,3-dienes6 or allenes7 instead of alkynes was also reported. Nevertheless, the use of alkenes has been rare, which is probably due to the difficulty of both the simultaneous coordination of these two components to nickel(0) and the subsequent oxidative cyclization.2e Thus far, the use of alkenes has been limited to the highly reactive ones such as methylenecyclopropane,8 norbornene9 and tetrafluoroethylene.10 Herein, we report an intramolecular reductive coupling reaction of simple alkenes and aldehydes with hydrosilanes catalyzed by nickel(0)/N-heterocyclic carbene (NHC), yielding a variety of silyl-protected 1-indanol derivatives in a diastereoselective manner (Scheme 1b). We also demonstrate the corresponding reductive coupling reaction with ketones instead of aldehydes.11
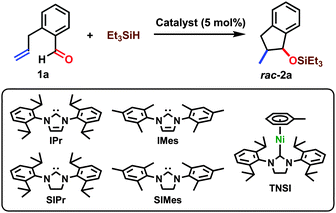
The reaction conditions were optimized through the use of o-allylbenzaldehyde (1a) and triethylsilane (Table 1). The formation of 1-triethylsiloxy-2-methylindan (2a) was confirmed when NHC ligands were employed, whereas PPh3 and PCy3 were not effective (entries 1–6). Among the NHCs we examined, SIPr gave 2a in a better yield at room temperature (69%, entry 5). In this reaction system, (η6-toluene)Ni(SIPr) (TNSI) was generated as a precursor for an active nickel(0)/SIPr catalyst.12 Indeed, the reaction conducted with TNSI provided 2a without a loss of the yield compared with the system comprising Ni(cod)2 and SIPr (entry 7 vs. 5). We thus used TNSI for the further optimization since TNSI can be prepared using a single-step, one-pot, and gram-scale method,12 and it readily dissolves in a variety of solvents including alkane media, unlike Ni(cod)2. In addition, the experimental manipulations were simplified and made more accurate by using the prepared nickel(0) complex. The reaction at 40 °C resulted in improvements in both yield and diastereoselectivity giving 2a in 99% isolated yield (entry 8). Among the various solvents we examined, toluene gave the highest yield and diastereoselectivity (entries 8–11). The syn-conformation between the silyl ether and the methyl groups in 2a was confirmed by comparison with reports in the literature after desilylation using TBAF (94% isolated yield).13,14
| Entry | Catalyst | Solvent | Temp. (°C) | Time (h) | Yieldb (%) | drb |
|---|---|---|---|---|---|---|
| a Employment of 5 mol% of Ni(cod)2 and 10 mol% of phosphine ligands. b Determined by GC using n-pentadecane as an internal standard. c Isolated yield. | ||||||
| 1a | Ni(cod)2/PCy3 | Toluene | rt | 24 | <1 | — |
| 2a | Ni(cod)2/PPh3 | Toluene | rt | 24 | <1 | — |
| 3 | Ni(cod)2/IPr | Toluene | rt | 24 | 14 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 4 | Ni(cod)2/IMes | Toluene | rt | 24 | 5 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 5 | Ni(cod)2/SIPr | Toluene | rt | 24 | 69 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 6 | Ni(cod)2/SIMes | Toluene | rt | 24 | 20 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 7 | TNSI | Toluene | rt | 24 | 69 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 8 | TNSI | Toluene | 40 | 12 | 94 (99)c | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | TNSI | THF | 40 | 12 | 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | TNSI | 1,4-Dioxane | 40 | 12 | 92 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | TNSI | Cyclohexane | 40 | 12 | 80 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
The impact of the steric and electronic nature of silanes on both the yield and diastereoselectivity was not significant (Table 2). Good to excellent yields and diastereoselectivities were obtained across a broad range of silanes, with the exception of (EtO)3SiH (entry 7). Other reducing reagents such as diethylzinc or triethylborane were less effective in this reaction.14
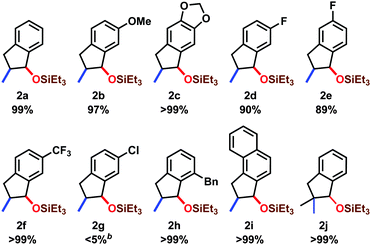
The scope of the reaction was investigated with respect to o-allylbenzaldehyde derivatives (1a–j) (Table 3). When electron-donating groups were bonded to the benzene ring (1b–c), the reaction proceeded well to give the corresponding silyl-protected 1-indanol derivatives in >97% isolated yields. The fluorine substituted products (2d–f) were obtained in >89% isolated yields; however, a trace amount of chlorine-substituted product 2g was obtained. Both products with the o-benzyl group (2h) and the naphthyl structure (2i) were obtained in >99% isolated yields. In all of these reactions, the syn-isomer was obtained with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The substituted allyl group was also employed to afford 2j in >99% isolated yield.
1 dr. The substituted allyl group was also employed to afford 2j in >99% isolated yield.
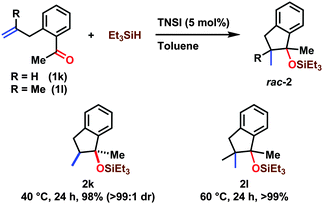
The results of a reductive coupling of alkenes and ketones with triethylsilane are shown in Scheme 2. The reaction of o-allylacetophenone (1k) took place efficiently to afford the corresponding silyl-protected 1-methyl-1-indanol (2k) in 98% isolated yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The syn-conformation between the silyl ether and the methyl groups in 2k was confirmed by comparison with reports in the literature after desilylation using TBAF.14,15 The substituted allyl group was also employed at 60 °C, giving 2l in >99% isolated yield.
1 dr. The syn-conformation between the silyl ether and the methyl groups in 2k was confirmed by comparison with reports in the literature after desilylation using TBAF.14,15 The substituted allyl group was also employed at 60 °C, giving 2l in >99% isolated yield.
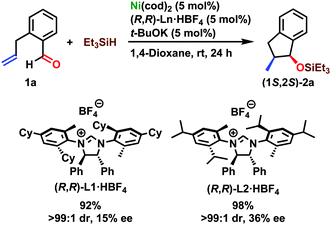
Preliminary results of the expansion of the presented reaction to an enantioselective version are given in Scheme 3.14 Chiral NHC ligands generated in situ by treating the imidazolinium salts (Ln·HBF4) with t-BuOK were applied in this reaction. The reductive coupling of 1a with triethylsilane was catalyzed by 5 mol% of Ni(cod)2 and L1 at room temperature for 24 h in 1,4-dioxane and provided 2a in 92% isolated yield, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 15% ee. The enantiomeric excess of 2a was determined using supercritical fluid chromatography (SFC) after converting it into 2-methyl-1-indanol (3a) through desilylation using TBAF.14 A novel ligand L2 was prepared according to the procedures of L1 reported by Montgomery et al.,16 and the system utilizing L2 afforded further improved results (98% isolated yield, >99
1 dr, and 15% ee. The enantiomeric excess of 2a was determined using supercritical fluid chromatography (SFC) after converting it into 2-methyl-1-indanol (3a) through desilylation using TBAF.14 A novel ligand L2 was prepared according to the procedures of L1 reported by Montgomery et al.,16 and the system utilizing L2 afforded further improved results (98% isolated yield, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 38% ee). The absolute configuration of (1S,2S)-2a was confirmed by comparing the specific rotation of the corresponding desilylation product (1S,2S)-3a with data found in the literature.13b
1 dr, and 38% ee). The absolute configuration of (1S,2S)-2a was confirmed by comparing the specific rotation of the corresponding desilylation product (1S,2S)-3a with data found in the literature.13b
This intramolecular reductive coupling might proceed through oxidative cyclization of an aldehyde and an alkene affording an oxanickelacycle intermediate as shown in Scheme 1.17 The oxidative cyclization process would be promoted by a Lewis acidic silane reagent.2e,3a The subsequent σ-bond metathesis and reductive elimination might give the product. Analogous mechanisms proceeding through oxanickelacycle intermediates were proposed in the reductive coupling reaction of aldehydes and alkynes.18
In conclusion, the nickel(0)/NHC-catalyzed diastereoselective intramolecular reductive coupling of alkenes and aldehydes with hydrosilanes was demonstrated. A variety of silyl-protected 1-indanol derivatives were prepared in excellent yields with perfect diastereoselectivities. The corresponding reductive coupling with ketones was also achieved. The preliminary results on the enantioselective reductive coupling were shown, in which the target indanol was obtained with a perfect diastereoselectivity and a promising enantioselectivity. Further development of this enantioselective process is ongoing in our group.
This work was supported by Grants-in-Aid for Young Scientists (A) (25708018) and Grants-in-Aid for Scientific Research on Innovative Area “Precise Formation of a Catalyst Having a Specified Field for Use in Extremely Difficult Substrate Conversion Reactions” (15H05803) and “Stimuli-responsive Chemical Species” (15H00943) from MEXT and by ACT-C from JST. Y. H. acknowledges support from the Frontier Research Base for Global Young Researchers, Osaka University, on the Program of MEXT. Y. H. expresses her special thanks for the Research Fellowship for Young Scientists from JSPS.
Notes and references
- For selected recent reviews and books on the nickel(0)-catalyzed reactions via nickelacycle intermediates, see: (a) M. Ohashi, Y. Hoshimoto and S. Ogoshi, Dalton Trans., 2015, 44, 12060 RSC; (b) Y. Hoshimoto, M. Ohashi and S. Ogoshi, Acc. Chem. Res., 2015, 48, 1746 CrossRef CAS PubMed; (c) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed; (d) J. Montgomery, Organonickel Chemistry, in Organometallics in Synthesis: Fourth Manual, ed. B. H. Lipshutz, Wiley, Hoboken, NJ, 2013, pp. 319–428 Search PubMed; (e) J. C. Leung and M. J. Krische, Chem. Sci., 2012, 3, 2202 RSC; (f) Modern Organonickel Chemistry, ed. Y. Tamaru, Wiely-VCH, Weinheim, 2005 Search PubMed.
- For selected recent reviews on nickel(0)-catalyzed reductive coupling reactions, see: (a) E. A. Standley, S. Z. Tasker, K. L. Jensen and T. F. Jamison, Acc. Chem. Res., 2015, 48, 1503 CrossRef CAS PubMed; (b) E. P. Jackson, H. A. Malik, G. J. Sormunen, R. D. Baxter, P. Liu, H. Wang, A.-R. Shareef and J. Montgomery, Acc. Chem. Res., 2015, 48, 1736 CrossRef CAS PubMed; (c) K. Tanaka and Y. Tajima, Eur. J. Org. Chem., 2012, 3715 CrossRef CAS; (d) M. Jeganmohan and C.-H. Cheng, Chem. – Eur. J., 2008, 14, 10876 CrossRef CAS PubMed; (e) S.-S. Ng, C.-Y. Ho, K. D. Schleicher and T. F. Jamison, Pure Appl. Chem., 2008, 80, 929 CrossRef CAS PubMed; (f) R. M. Moslin, K. Miller-Moslin and T. F. Jamison, Chem. Commun., 2007, 4441 RSC; (g) J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS PubMed; (h) S.-i. Ikeda, Angew. Chem., Int. Ed., 2003, 42, 5120 CrossRef CAS PubMed; (i) J. Montgomery, Acc. Chem. Res., 2000, 33, 467 CrossRef CAS PubMed.
- (a) S. Ogoshi, M.-a. Oka and H. Kurosawa, J. Am. Chem. Soc., 2004, 126, 11802 CrossRef CAS PubMed; (b) Y. Hoshimoto, Y. Hayashi, H. Suzuki, M. Ohashi and S. Ogoshi, Angew. Chem., Int. Ed., 2012, 51, 10812 CrossRef CAS PubMed.
- (a) C.-Y. Zhou, S.-F. Zhu, L.-X. Wang and Q.-L. Zhou, J. Am. Chem. Soc., 2010, 132, 10955 CrossRef CAS PubMed; (b) K. M. Miller, W.-S. Huang and T. F. Jamison, J. Am. Chem. Soc., 2003, 125, 3442 CrossRef CAS PubMed; (c) W.-S. Huang, J. Chan and T. F. Jamison, Org. Lett., 2000, 2, 4221 CrossRef CAS PubMed; (d) X.-Q. Tang and J. Montgomery, J. Am. Chem. Soc., 2000, 122, 6950 CrossRef CAS; (e) X.-Q. Tang and J. Montgomery, J. Am. Chem. Soc., 1999, 121, 6098 CrossRef CAS; (f) E. Oblinger and J. Montgomery, J. Am. Chem. Soc., 1997, 119, 9065 CrossRef CAS.
- Nickel(0)-catalyzed reductive coupling of 1,6-enynes and aldehydes, see: R. M. Moslin, K. M. Miller and T. F. Jamison, Tetrahedron, 2006, 62, 7598 CrossRef CAS and references therein.
- N. Saito, Y. Sugimura and Y. Sato, Org. Lett., 2010, 12, 3494 CrossRef CAS PubMed and references therein.
- (a) S.-S. Ng and T. F. Jamison, J. Am. Chem. Soc., 2005, 127, 7320 CrossRef CAS PubMed; (b) S.-K. Kang and S.-K. Yoon, Chem. Commun., 2002, 2634 RSC; (c) J. Montgomery and M. Song, Org. Lett., 2002, 4, 4009 CrossRef CAS PubMed.
- K. Ogata, Y. Atsuumi and S.-i. Fukuzawa, Org. Lett., 2010, 12, 4536 CrossRef CAS PubMed.
- (a) K. Ogata, A. Toh, D. Shimada and S.-i. Fukuzawa, Chem. Lett., 2012, 41, 157 CrossRef CAS; (b) K. Ogata, Y. Atsuumi, D. Shimada and S.-i. Fukuzawa, Angew. Chem., Int. Ed., 2011, 50, 5896 CrossRef CAS PubMed.
- (a) M. Ohashi, T. Kawashima, T. Taniguchi, K. Kikushima and S. Ogoshi, Organometallics, 2015, 34, 1604 CrossRef CAS; (b) M. Ohashi, H. Shirataki, K. Kikushima and S. Ogoshi, J. Am. Chem. Soc., 2015, 137, 6496 CrossRef CAS PubMed.
- Nickel(0)-catalyzed reductive coupling with ketones, see ref. 6 and: (a) W. Fu, M. Nie, A. Wang, Z. Cao and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 2520 CrossRef CAS PubMed; (b) K. M. Miller and T. F. Jamison, Org. Lett., 2005, 7, 3077 CrossRef CAS PubMed; (c) J. Chan and T. F. Jamison, J. Am. Chem. Soc., 2004, 126, 10682 CrossRef CAS PubMed.
- Y. Hoshimoto, Y. Hayashi, H. Suzuki, M. Ohashi and S. Ogoshi, Organometallics, 2014, 33, 1276 CrossRef CAS.
- (a) P. A. Marshall and R. H. Prager, Aust. J. Chem., 1979, 32, 1251 CrossRef CAS; (b) R. Fernández, A. Ros, A. Magriz, H. Dietrich and J. M. Lassaletta, Tetrahedron, 2007, 63, 6755 CrossRef.
- See the ESI† for details.
- N. M. Kablaoui and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 3182 CrossRef CAS.
- M. R. Chaulagain, G. J. Sormunen and J. Montgomery, J. Am. Chem. Soc., 2007, 129, 9568 CrossRef CAS PubMed.
- A mechanism initiated by oxidative addition of hydrosilane to nickel(0) yielding a nickel(II) hydrido silyl complex is unlikely. Stoichiometric treatment of hydrosilane with TNSI was examined at room temperature and no observable change was confirmed by NMR spectroscopy.
- (a) E. P. Jackson and J. Montgomery, J. Am. Chem. Soc., 2015, 137, 958 CrossRef CAS PubMed; (b) M. T. Haynes II, P. Liu, R. D. Baxter, A. J. Nett, K. N. Houk and J. Montgomery, J. Am. Chem. Soc., 2014, 136, 17495 CrossRef PubMed; (c) P. Liu, P. McCarren, P. H.-Y. Cheong, T. F. Jamison and K. N. Houk, J. Am. Chem. Soc., 2010, 132, 2050 CrossRef CAS PubMed; (d) N. Saito, T. Katayama and Y. Sato, Org. Lett., 2008, 10, 3829 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c6cc01915c |
| This journal is © The Royal Society of Chemistry 2016 |