Furanosic forms of sugars: conformational equilibrium of methyl β-d-ribofuranoside†
Abstract
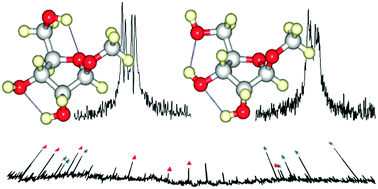
The investigation of an isolated ribofuranose unit in the gas phase reveals the intrinsic conformational landscape of the biologically active sugar form. We report the rotational spectra of two conformers of methyl β-D-ribofuranoside in a supersonic jet expansion. Both conformers adopt a near twisted (3T2) ring conformation with the methoxy and hydroxymethyl substituents involved in various intramolecular hydrogen bonds.

 Please wait while we load your content...
Please wait while we load your content...