Open Access Article
Open Access ArticleChiral-at-metal iridium complex for efficient enantioselective transfer hydrogenation of ketones†
Cheng
Tian
a,
Lei
Gong
*a and
Eric
Meggers
*ab
aCollege of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, People's Republic of China. E-mail: gongl@xmu.edu.cn; meggers@chemie.uni-marburg.de
bPhilipps-Universität Marburg, Hans-Meerwein-Strasse 4, 35043 Marburg, Germany
First published on 22nd February 2016
Abstract
A bis-cyclometalated iridium(III) complex with metal-centered chirality catalyzes the enantioselective transfer hydrogenation of ketones with high enantioselectivities at low catalyst loadings down to 0.002 mol%. Importantly, the rate of catalysis and enantioselectivity are markedly improved in the presence of a pyrazole co-ligand. The reaction is proposed to proceed via an iridium-hydride intermediate exploiting metal–ligand cooperativity (bifunctional catalysis).
Transition metal catalyzed asymmetric transfer hydrogenation (ATH) has developed into a popular method for the generation of non-racemic chiral alcohols and amines using isopropanol, formic acid/triethylamine or sodium formate as convenient and inexpensive hydrogen sources.1 Since Noyori's seminal discovery of highly enantioselective ATH catalysts based on ruthenium(II) half sandwich complexes containing monotosylated 1,2-diamines,2 transition metals such as Ru(II),3 Os(II),4 Ir(III),5 Rh(III),6 and Fe(II)7 have been combined with a large variety of different chiral ligands to achieve high turnover numbers (TON) and turnover frequencies (TOF) for different substrate classes.8 Here we report a unique catalyst that relies on metal-centered chirality using exclusively achiral ligands.9
We recently developed a novel class of chiral Lewis acid catalysts based on octahedral chiral-only-at-metal iridium(III) and rhodium(III) complexes, in which the octahedral metal center is coordinated irreversibly by two cyclometalating bidentate ligands in a propeller-type fashion, complemented by two exchange-labile acetonitriles.10–17 In these catalysts, metal-centered chirality (metal centrochirality) is the only source of chirality. We were wondering if such complexes are suitable for catalyzing ATH and we used the reduction of acetophenone as our initial model reaction (1a → 2a, Fig. 1).
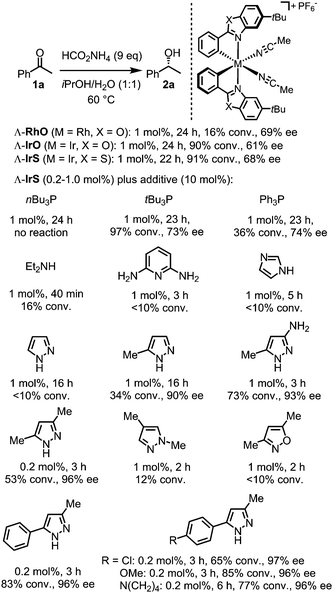 | ||
| Fig. 1 Initial experiments and screening of ligand additives. Conversion determined by 1H-NMR and enantioselectivity by HPLC on a chiral stationary phase. | ||
Using ammonium formate as the hydrogen source, the rhodium complex Λ-RhO (1 mol%) catalyzed the reduction of acetophenone only sluggishly, providing just 16% conversion and 69% ee after 24 hours at 60 °C, whereas the higher congener Λ-IrO (1 mol%) gave better results with 90% conversion after 24 hours and 61% ee. Replacing the benzoxazole ligands (Λ-IrO) against benzothiazole (Λ-IrS) further improved the outcome reaching 91% conversion after 22 hours with 68% ee. We therefore chose Λ-IrS as the catalyst of choice and next investigated the effect of additional monodentate ligands on the catalysis. The results are shown in Fig. 1. Whereas some ligands such as nBu3P, 2,6-diaminopyridine or imidazole suppressed the catalytic activity, others such as tBu3P resulted in a slight improvement of the enantioselectivity. To our delight, 3,5-dimethylpyrazole markedly improved the catalytic activity and enantioselectivity. With a reduced catalyst loading of just 0.2 mol%, 3 hours reaction time resulted in a conversion of 53% with 96% ee. Replacing the methyl group at the 5-position with a phenyl moiety further improved the results with the best compromise out of reaction rate and enantioselectivity achieved with 5-(4-methoxyphenyl)-3-methyl-1H-pyrazole.
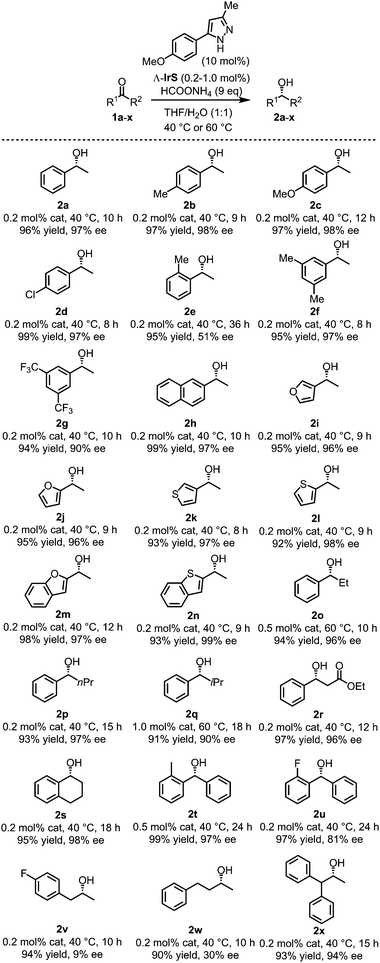
We used the combination of Λ-IrS (0.2–1.0 mol%) and the best pyrazole additive (10 mol%) for investigating the substrate scope (1a–x) under optimized conditions in THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent. Fig. 2 reveals that the ATH reaction of acetophenones with electron donating or withdrawing substitutents within the phenyl moiety provided both high yields and good enantioselectivities (products 2a–d,f,g) with an exception of the ortho-methyl substituted substrate (product 2e, 51% ee). Typically, electron withdrawing groups were slightly less beneficial with regard to enantioselectivity, which might be due to some contribution from uncatalyzed background reaction. Other aromatic ketones containing a naphthyl moiety (product 2h), heteroaromatic ring (products 2i–n), larger aliphatic groups (products 2o–q), or an additional ester functionality (product 2r), as well as a cyclic ketone (product 2s) were all well converted. Diaryl ketones also provided satisfactory results (products 2t,u). As for dialkyl ketones bearing two primary alkyl chains, for example affording the alcohols 2v and 2w, high yields (90–94%) while low ee values (9% and 30% ee, respectively) were achieved. However, a substrate with one bulky secondary alkyl substituent afforded the desired alcohol 2x with 93% yield and 94% ee within 15 hours, suggesting that aliphatic ketones could also work nicely for selected cases. In addition, it is worth to mention that the reaction can be scaled up. For example, 1.0 g of phenyl(o-tolyl)methanone produced 1.0 g of its corresponding alcohol 2t (yield 99%) with 97% ee in presence of 0.5 mol% catalyst.‡
1) as the solvent. Fig. 2 reveals that the ATH reaction of acetophenones with electron donating or withdrawing substitutents within the phenyl moiety provided both high yields and good enantioselectivities (products 2a–d,f,g) with an exception of the ortho-methyl substituted substrate (product 2e, 51% ee). Typically, electron withdrawing groups were slightly less beneficial with regard to enantioselectivity, which might be due to some contribution from uncatalyzed background reaction. Other aromatic ketones containing a naphthyl moiety (product 2h), heteroaromatic ring (products 2i–n), larger aliphatic groups (products 2o–q), or an additional ester functionality (product 2r), as well as a cyclic ketone (product 2s) were all well converted. Diaryl ketones also provided satisfactory results (products 2t,u). As for dialkyl ketones bearing two primary alkyl chains, for example affording the alcohols 2v and 2w, high yields (90–94%) while low ee values (9% and 30% ee, respectively) were achieved. However, a substrate with one bulky secondary alkyl substituent afforded the desired alcohol 2x with 93% yield and 94% ee within 15 hours, suggesting that aliphatic ketones could also work nicely for selected cases. In addition, it is worth to mention that the reaction can be scaled up. For example, 1.0 g of phenyl(o-tolyl)methanone produced 1.0 g of its corresponding alcohol 2t (yield 99%) with 97% ee in presence of 0.5 mol% catalyst.‡
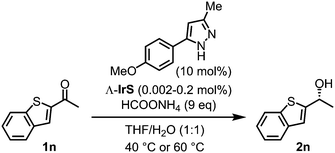
Next, we chose 2-acetyl benzothiophene for testing catalytic performance of the Λ-IrS/monodentate pyrazole system at lower catalyst loadings (1n → 2n). As illustrated in Table 1, the catalyst loading could be reduced to 0.005 mol% (S/C = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) while still keeping a satisfactory reaction time of 108 hours for complete conversion at 60 °C without affecting the enantioselectivity (entries 1–5). A further reduction to 0.002 mol% catalyst led to a slight drop in enantioselectivity value (96.6% ee) while a significantly lower reaction rate prevented full conversion (entry 6).
000) while still keeping a satisfactory reaction time of 108 hours for complete conversion at 60 °C without affecting the enantioselectivity (entries 1–5). A further reduction to 0.002 mol% catalyst led to a slight drop in enantioselectivity value (96.6% ee) while a significantly lower reaction rate prevented full conversion (entry 6).
| Entry | Cat. loading (mol%) | T (°C) | t (h) | Conv.b (%) | eec (%) |
|---|---|---|---|---|---|
a Reaction conditions: A mixture of Λ-IrS (0.002–0.2 mol%), 5-(4-methoxyphenyl)-3-methyl-1H-pyrazole (0.066 mmol), and HCOONH4 (6.0 mmol) in THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.67 mL) was stirred at room temperature for 10 min before the substrate 2-acetyl benzothiophene (0.66 mmol) was added and the solution stirred at 40 or 60 °C for the indicated time.
b Conversion determined by 1H-NMR.
c Enantioselectivity by HPLC on a chiral stationary phase.
d Values in bracket for a reaction time of 240 hours. 1) (0.67 mL) was stirred at room temperature for 10 min before the substrate 2-acetyl benzothiophene (0.66 mmol) was added and the solution stirred at 40 or 60 °C for the indicated time.
b Conversion determined by 1H-NMR.
c Enantioselectivity by HPLC on a chiral stationary phase.
d Values in bracket for a reaction time of 240 hours.
|
|||||
| 1 | 0.2 | 40 | 9 | Quant. | 98.7 |
| 2 | 0.05 | 40 | 30 | Quant. | 98.7 |
| 3 | 0.01 | 40 | 144 | Quant. | 98.7 |
| 4 | 0.01 | 60 | 48 | Quant. | 98.4 |
| 5 | 0.005 | 60 | 108 | Quant. | 98.4 |
| 6d | 0.002 | 60 | 120 (240) | 51 (56) | 96.6 (96.6) |
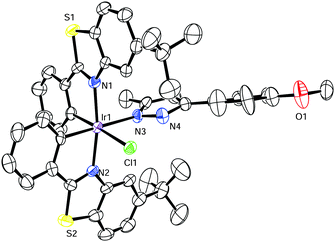
Mechanistically, we propose that the precatalyst Λ-IrS bearing labile acetonitriles undergoes fast ligand exchange with one pyrazole molecule, followed by reaction with ammonium formate to generate an active iridium hydride species. With assistance of the ancillary pyrazole ligand, the subsequent concerted transfer of a hydride to the carbonyl carbon and a proton to the carbonyl oxygen leads to formation of the chiral secondary alcohol. The effective asymmetric induction can be explained by less steric hindrance in the favored transition state as well as additional π–π stacking between the aromatic ring of the substrate and one cyclometalating benzothiazoline moiety of the iridium complex (Fig. 3, left side). The high catalytic efficiency is in parts attributed to the rigid structure of the catalyst and intermediate limiting the degree of conformational flexibility, thereby providing entropic advantages during catalysis. The mechanism is consistent with an observed R-configuration of the formed chiral alcohol assigned by comparison of optical rotations with published examples.8e,18 Additionally, the importance of the ancillary pyrazole ligand with the crucial role of the N–H group is supported by the sluggish results achieved with closely related additive ligands which lack this N–H, such as 1,4-dimethyl-1H-pyrazole or 3,5-dimethylisoxazole (Fig. 1).19 A crystal structure of 5-(4-methoxyphenyl)-3-methyl-1H-pyrazole coordinated to the bis-cyclometalated iridium complex confirms that the pyrazole prefers a conformation in which the N–H group is in a perfect position for the proposed bifunctional catalysis (Fig. 4).20
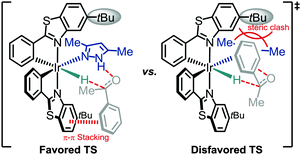 | ||
| Fig. 3 Proposed transition states through the association of the substrate with a pyrazole-coordinated iridium hydride intermediate explaining the observed enantioselectivities. | ||
In conclusion, we here reported a highly efficient asymmetric transfer hydrogenation for ketones catalyzed by a bis-cyclometalated chiral-at-metal iridium(III) complex in the presence of an additional pyrazole ligand. The reaction is proposed to proceed through an iridium-hydride intermediate exploiting metal–ligand cooperativity involving the coordinated pyrazole ligand. A variety of aryl ketones and even one aliphatic ketone are well tolerated in the ATH reaction by affording the secondary alcohols with good to excellent enantioselectivities at catalyst loadings down to 0.002 mol%. Applications to other substrate classes such as imines are ongoing in our laboratory.
We thank the National Natural Science Foundation of P. R. China (grant no. 21272192, 21472154 and 21572184), the Program for Changjiang Scholars and Innovative Research Team of P. R. China (PCSIRT), the National Thousand Talents Program of P. R. China, the Fundamental Research Funds for the Central Universities (grant no. 20720160027), and the 985 Program of the Chemistry and Chemical Engineering disciplines of Xiamen University.
Notes and references
- For selected recent reviews on asymmetric transfer hydrogenation of ketones, see: (a) S. Gladiali and E. Alberico, Chem. Soc. Rev., 2006, 35, 226–236 RSC; (b) X. Wu and J. Xiao, Chem. Commun., 2007, 2449–2466 RSC; (c) X. Wu, C. Wang and J. Xiao, Platinum Met. Rev., 2010, 54, 3–19 CrossRef CAS; (d) R. Malacea, R. Poli and E. Manoury, Coord. Chem. Rev., 2010, 254, 729–752 CrossRef CAS; (e) A. Bartoszewicz, N. Ahlsten and B. Martin-Matute, Chem. – Eur. J., 2013, 19, 7274–7302 CrossRef CAS PubMed; (f) J. Ito and H. Nishiyama, Tetrahedron Lett., 2014, 55, 3133–3146 CrossRef; (g) P. E. Sues, K. Z. Demmans and R. H. Morris, Dalton Trans., 2014, 43, 7650–7667 RSC; (h) J. Václavík, P. Sot, J. Pecháček, B. Vilhanová, O. Matuška, M. Kuzma and P. Kačer, Molecules, 2014, 19, 6987–7007 CrossRef PubMed; (i) Y.-Y. Li, S.-L. Yu, W.-Y. Shen and J.-X. Gao, Acc. Chem. Res., 2015, 48, 2587–2598 CrossRef CAS PubMed.
- S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 7562–7563 CrossRef CAS.
- For selected examples of ruthenium(II) catalyzed ATH reactions, see: (a) T. Cheng, Q. Ye, Q. Zhao and G. Liu, Org. Lett., 2015, 17, 4972–4975 CrossRef CAS PubMed; (b) T. Slagbrand, T. Kivijārvi and H. Adolfsson, ChemCatChem, 2015, 7, 3445–3449 CrossRef CAS; (c) A. Kišić, M. Stephan and B. Mohar, Adv. Synth. Catal., 2015, 357, 2540–2546 CrossRef; (d) R. Soni, T. H. Hall, B. P. Mitchell, M. R. Owen and M. Wills, J. Org. Chem., 2015, 80, 6784–6793 CrossRef CAS PubMed; (e) Z. Xu, Y. Li, J. Liu, N. Wu, K. Li, S. Zhu, R. Zhang and Y. Liu, Org. Biomol. Chem., 2015, 13, 7513–7516 RSC; (f) C. Cesari, A. Cingolani, C. Parise, S. Zacchini, V. Zanotti, M. C. Cassani and R. Mazzoni, RSC Adv., 2015, 5, 94707–94718 RSC; (g) A. Matsuoka, C. A. Sandoval, M. Uchiyama, R. Noyori and H. Naka, Chem. – Asian J., 2015, 10, 112–115 CrossRef CAS PubMed; (h) C. Kucukturkmen, A. Agac, A. Eren, I. Karakaya, M. Aslantas, O. Celik, S. Ulukanli and S. Karabuga, Catal. Commun., 2016, 74, 122–125 CrossRef CAS.
- For selected examples of osmium(II) catalyzed ATH reactions, see: (a) D. Carmona, F. J. Lahoz, P. García-Orduña, L. A. Oro, M. P. Lamata and F. Viguri, Organometallics, 2012, 31, 3333–3345 CrossRef CAS; (b) E. Vega, E. Lastra and M. P. Gamasa, Inorg. Chem., 2013, 52, 6193–6198 CrossRef CAS PubMed; (c) J. P. C. Coverdale, C. Sanchez-Cano, G. J. Clarkson, R. Soni, M. Wills and P. J. Sadler, Chem. – Eur. J., 2015, 21, 8043–8046 CrossRef CAS PubMed; (d) A. Bolje, S. Hohloch, M. van der Meer, J. Kosmrlj and B. Sarkar, Chem. – Eur. J., 2015, 21, 6756–6764 CrossRef CAS PubMed.
- For selected examples of iridium(III) catalyzed ATH reactions, see: (a) D. Zerla, G. Facchetti, M. Fusè, M. Pellizzoni, C. Castellano, E. Cesarotti, R. Gandolfi and I. Rimoldi, Tetrahedron: Asymmetry, 2014, 25, 1031–1037 CrossRef CAS; (b) D. M. Morris, M. McGeagh, D. De Peña and J. S. Merola, Polyhedron, 2014, 84, 120–135 CrossRef CAS; (c) K. Yoshida, T. Kamimura, H. Kuwabara and A. Yanagisawa, Chem. Commun., 2015, 51, 15442–15445 RSC; (d) A. Matsunami, Y. Kayaki and T. Ikariya, Chem. – Eur. J., 2015, 21, 13513–13517 CrossRef CAS PubMed; (e) B. Ak, M. Aydemir, F. Durap, N. Meriç, D. Elma and A. Baysal, Tetrahedron: Asymmetry, 2015, 26, 1307–1313 CrossRef CAS; (f) Z.-Q. Rong, Y. Zhang, R. H. B. Chua, H.-J. Pan and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 4944–4947 CrossRef CAS PubMed; (g) J. M. M. Verkade, P. J. L. M. Quaedflieg, G. K. M. Verzijl, L. Lefort, F. L. van Delft, J. G. de Vries and F. P. J. T. Rutjes, Chem. Commun., 2015, 51, 14462–14464 RSC; (h) W.-P. Liu, M.-L. Yuan, X.-H. Yang, K. Li, J.-H. Xie and Q.-L. Zhou, Chem. Commun., 2015, 51, 6123–6125 RSC.
- For selected examples of rhodium(III) catalyzed ATH reactions, see: (a) L. Zhang, R. Qiu, X. Xue, Y. Pan, C. Xu, H. Li and L. Xu, Adv. Synth. Catal., 2015, 357, 3529–3537 CrossRef CAS; (b) K. Farrell, H. Müller-Bunz and M. Albrecht, Organometallics, 2015, 34, 5723–5733 CrossRef CAS; (c) J. Lu, J. Dimroth and M. Weck, J. Am. Chem. Soc., 2015, 137, 12984–12989 CrossRef CAS PubMed; (d) P.-G. Echeverria, C. Férard, P. Phansavath and V. Ratovelomanana-Vidal, Catal. Commun., 2015, 62, 95–99 CrossRef CAS; (e) Z. Lin, J. Li, Q. Huang, Q. Huang, Q. Wang, L. Tang, D. Gong, J. Yang, J. Zhu and J. Deng, J. Org. Chem., 2015, 80, 4419–4429 CrossRef CAS PubMed; (f) V. S. Shende, S. H. Deshpande, S. K. Shingote, A. Joseph and A. A. Kelkar, Org. Lett., 2015, 17, 2878–2881 CrossRef CAS PubMed.
- For selected examples of iron(II) catalyzed ATH reactions, see: (a) W. Zuo, A. J. Lough, Y. F. Li and R. H. Morris, Science, 2013, 342, 1080–1083 CrossRef CAS PubMed; (b) R. Bigler and A. Mezzetti, Org. Lett., 2014, 16, 6460–6463 CrossRef CAS PubMed; (c) W. Zuo, S. Tauer, D. E. Prokopchuk and R. H. Morris, Organometallics, 2014, 33, 5791–5801 CrossRef CAS; (d) S. A. M. Smith and R. H. Morris, Synthesis, 2015, 1775–1779 CAS; (e) R. Bigler, R. Huber and A. Mezzetti, Angew. Chem., Int. Ed., 2015, 54, 5171–5174 CrossRef CAS PubMed; (f) W. Zuo and R. H. Morris, Nat. Protoc., 2015, 10, 241–257 CrossRef CAS PubMed.
- For selected examples of ATH reactions catalyzed by other transition metals, see: (a) K. Saito, Y. Kajiwara and T. Akiyama, Angew. Chem., Int. Ed., 2013, 52, 13284–13288 CrossRef CAS PubMed; (b) P. Clavero, A. Grabulosa, M. Font-Bardia and G. Muller, J. Mol. Catal. A: Chem., 2014, 391, 183–190 CrossRef CAS; (c) S. Guo, P. Yang and J. Zhou, Chem. Commun., 2015, 51, 12115–12117 RSC; (d) H. Xu, P. Yang, P. Chuanprasit, H. Hirao and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 5112–5116 CrossRef CAS PubMed; (e) J. Guo, J. Chen and Z. Lu, Chem. Commun., 2015, 51, 5725–5727 RSC; (f) A. Keßberg and P. Metz, Angew. Chem., Int. Ed., 2016, 55, 1160–1163 CrossRef PubMed.
- For reviews covering chiral-at-metal complexes in catalysis, see: (a) H. Brunner, Angew. Chem., Int. Ed., 1999, 38, 1194–1208 CrossRef CAS; (b) P. D. Knight and P. Scott, Coord. Chem. Rev., 2003, 242, 125–143 CrossRef CAS; (c) M. Fontecave, O. Hamelin and S. Ménage, Top. Organomet. Chem., 2005, 15, 271–288 CrossRef CAS; (d) E. B. Bauer, Chem. Soc. Rev., 2012, 41, 3153–3167 RSC; (e) L. Gong, L.-A. Chen and E. Meggers, Angew. Chem., Int. Ed., 2014, 53, 10868–10874 CrossRef CAS PubMed; (f) Z.-Y. Cao, W. D. G. Brittain, J. S. Fossey and F. Zhou, Catal. Sci. Technol., 2015, 5, 3441–3451 RSC.
- H. Huo, C. Fu, K. Harms and E. Meggers, J. Am. Chem. Soc., 2014, 136, 2990–2993 CrossRef CAS PubMed.
- H. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt and E. Meggers, Nature, 2014, 515, 100–103 CrossRef CAS PubMed.
- C. Wang, L.-A. Chen, H. Huo, X. Shen, K. Harms, L. Gong and E. Meggers, Chem. Sci., 2015, 6, 1094–1100 RSC.
- C. Wang, Y. Zheng, H. Huo, P. Röse, L. Zhang, K. Harms, G. Hilt and E. Meggers, Chem. – Eur. J., 2015, 21, 7355–7359 CrossRef CAS PubMed.
- X. Shen, H. Huo, C. Wang, B. Zhang, K. Harms and E. Meggers, Chem. – Eur. J., 2015, 21, 9720–9726 CrossRef CAS PubMed.
- Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045–13048 CrossRef CAS PubMed.
- Y. Huang, L. Song, L. Gong and E. Meggers, Chem. – Asian J., 2015, 10, 2738–2743 CrossRef CAS PubMed.
- C. Wang, J. Qin, X. Shen, R. Riedel, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 685–688 CrossRef CAS PubMed.
- (a) X. Ren, G. Li, S. Wei and H. Du, Org. Lett., 2015, 17, 990–993 CrossRef CAS PubMed; (b) S. Phothongkam and B. Uang, Asian J. Org. Chem., 2015, 4, 794–799 CrossRef CAS; (c) Z. Zuo, L. Zhang, X. Leng and Z. Huang, Chem. Commun., 2015, 51, 5073–5076 RSC.
- The slow conversion in the presence of the sterically less hindered 5-methyl-1H-pyrazole and pyrazole itself can be rationalized with a catalyst deactivation through double coordination.
- (a) T. Ikariya, K. Murata and R. Noyori, Org. Biomol. Chem., 2006, 4, 393–406 RSC; (b) T. Ikariya and A. J. Blacker, Acc. Chem. Res., 2007, 40, 1300–1308 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and analytical data. CCDC 1450140. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc00972g |
‡ Catalysis on gram-scale: To a biphasic solution of 5-(4-methoxyphenyl)-3-methyl-1H-pyrazole (95.9 mg, 0.51 mmol) and HCOONH4 (2.89 g, 45.9 mmol) in THF/H2O (2.57 mL/2.57 mL) was added the metal catalyst Λ-IrS (24.3 mg, 0.026 mmol) in a brown glass vial. The mixture was stirred for 10 min at room temperature, then phenyl(o-tolyl)methanone (1t, 1.00 g, 5.10 mmol) was added. The reaction solution was stirred at 40 °C for 30 h, cooled down to room temperature and then dried under high vacuum. The residue was purified by flash chromatography on silica gel (n-hexane/dichloromethane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1 1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford product 2t as a yellow solid (0.999 g, 5.07 mmol, yield: 99%). Enantiomeric excess of 97% ee was established by HPLC analysis (Chiralpak OJ column, 250 × 4.6 mm, absorbance at 220 nm, n-hexane/isopropanol = 95 2) to afford product 2t as a yellow solid (0.999 g, 5.07 mmol, yield: 99%). Enantiomeric excess of 97% ee was established by HPLC analysis (Chiralpak OJ column, 250 × 4.6 mm, absorbance at 220 nm, n-hexane/isopropanol = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, flow rate: 1.0 mL min−1, 25 °C, tr(minor) = 27.2 min, tr(major) = 30.8 min). [α]20D = +6.8 (c 1.0, CHCl3). 5, flow rate: 1.0 mL min−1, 25 °C, tr(minor) = 27.2 min, tr(major) = 30.8 min). [α]20D = +6.8 (c 1.0, CHCl3). |
| This journal is © The Royal Society of Chemistry 2016 |