Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
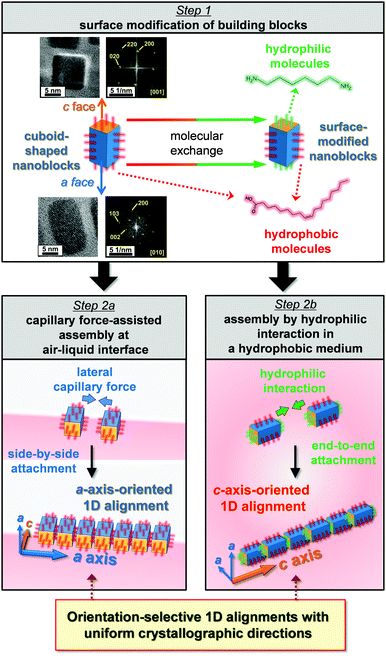
Orientation-selective alignments of nanoblocks in a and c directions of a tetragonal system through molecularly mediated manipulation†
Yoshitaka
Nakagawa
,
Hiroyuki
Kageyama
,
Yuya
Oaki
and
Hiroaki
Imai
*
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: hiroaki@applc.keio.ac.jp
First published on 17th March 2016
Abstract
Tetragonal Mn3O4 nanocuboids were aligned in selective crystallographic directions by molecularly mediated manipulation. Alignment in the a direction was produced by a face-by-a face assembly of hydrophobic nanocuboids covered with oleic acid. Another 1D array in the c direction was obtained through c face-to-c face assembly with the replacement of the organic modifiers.
Based on recent developments in colloidal chemistry, diverse nanometric crystals with well-defined morphologies, such as spheres,1 octahedra,2 and plates,3 have been successfully synthesized as uniform nanometric building blocks. Self-assembly of the inorganic nanocrystals into ordered architectures is a fascinating phenomenon as a bottom-up approach to achieve a wide range of novel functional materials. Well-aligned ensembles of the nanocrystals often exhibit interesting collective properties that are different from those displayed by individual nanocrystals and bulky large crystals.4,5 2D and 3D close-packed arrays of uniform spherical nanoparticles are built through self-assembly in liquid media.1,6 However, dimension-controlled assembly of spherical particles for 1D, 2D, and 3D arrangements is fundamentally difficult because of their shape isotropy.
Recently, nanocrystals with anisotropic shapes have attracted much attention as useful building blocks for the fabrication of various ordered assemblies. 1D, 2D, and 3D arrangements consisting of nanorods have been achieved through dimension-controlled assembly by means of their shape anisotropy.7–11 Moreover, two types of 1D arrays were obtained through side-by-side and end-to-end attachments of anisotropic nanoblocks, such as gold nanorods.12–15 However, the crystallographic directions of the building units in these assemblies were not aligned like polycrystalline structures because strictly oriented attachment through face-to-face contact was not achieved by nanorods that are not surrounded by well-defined faces.
The next challenge is how to manipulate the alignment of nanometric building blocks with uniform crystallographic directions, which is very important for controlling chemical and physical properties, such as ion permeability, catalytic properties, and magnetic properties. Single-crystalline architectures would be obtained by the epitaxial attachments of nanocrystals that are oriented in the same direction.16–18 This is an interesting approach to micrometric architectures with specifically designed shapes and crystallographic directions using nanometric blocks. One promising candidate for solving the problem is using rectangular nanoblocks, including nanocubes and nanocuboids that tend to assemble in the same crystallographic direction like a single crystal by facing well-defined facets. Nanocubes covered with amphiphilic molecules were synthesized from various cubic crystals of metals and metal oxides.19–30 A wide variety of 2D and 3D single-crystal-like superlattices are formed via the oriented self-assembly of the building blocks.22–25 Micrometer- and millimeter-scale assemblies consisting of the nanocubes have been fabricated on a substrate convective self-assembly using their dispersion.31–34 However, the regulation of the direction and dimension of the oriented self-assembly of isotropic nanocubes is basically hard without any external fields or complicated surface modification.35,36 On the other hand, the crystallographic orientations of 1D, 2D, and 3D ordered arrays consisting of manganese oxide nanocuboids have been sufficiently controlled for the fabrication of diversely shaped assemblies by means of shape anisotropy of building blocks.37 Single-crystalline 1D chains and 2D panels were achieved through the epitaxial attachments of crystallographically aligned nanocrystals.38 On the other hand, direction-selective 1D alignment of the anisotropic nanoblocks has not been achieved sufficiently by using systems composed of single building blocks and a single medium.
In the present work, 1D arrays elongated along the a and c axes were selectively fabricated using tetragonal Mn3O4 nanocuboids by means of crystallographic anisotropy of the building blocks through molecularly mediated manipulation. As shown in Fig. 1, surfaces of the building blocks were modified through selective molecular exchange by means of different degrees of adsorption of organic molecules (Step 1). Anisotropic nanoblocks covered with hydrophobic molecules were well-dispersed in hydrophobic dispersion. A 1D array elongated along the a direction was obtained through side-by-side (a face-by-a face) assembly of the anisotropic blocks with evaporation of the dispersion medium (Step 2a). Surface-modified nanoblocks that exposed hydrophilic c faces were assembled in hydrophobic dispersion by facing the hydrophilic faces. Another 1D array elongated along the c axis was then obtained in the hydrophobic medium by end-to-end (c face-to-c face) attachment (Step 2b). We regulated the crystallographic orientation of the alignment of the nanocrystals using a single hydrophobic dispersion medium and single building blocks. The face-selective exchange of the molecules adsorbed on the nanocuboids is essential for nanometric manipulation based on the hydrophobic–hydrophilic interaction.
Anisotropic rectangular nanoblocks of tetragonal Mn3O4 were synthesized using a two-phase solvothermal method reported in our previous work.37,39 As shown in Fig. 1, the fast Fourier transform (FFT) spots corresponding to the lattice fringes of tetragonal Mn3O4 (a = 0.576 nm and c = 0.944 nm) were observed by high-resolution transmission electron microscopy (HRTEM). The width and length of the nanocuboids that exhibited truncated cuboids with four {100} faces and two (001) faces were ca. 10 nm and ca. 20 nm, respectively. The presence of oleic acid covering the nanocuboids was confirmed by Raman spectroscopy.39 The terminal COO− of oleic acid is deduced to be attached to the surface of the Mn3O4 nanoblocks because the Mn3O4 nanoblocks were produced through oriented attachment of tiny nanoparticles of manganese compounds covered by dehydrogenated oleic acid in the aqueous phase.39 The hydrophobic nanoblocks were well dispersed in a hydrophobic dispersion medium, such as a mixture of toluene and hexane.
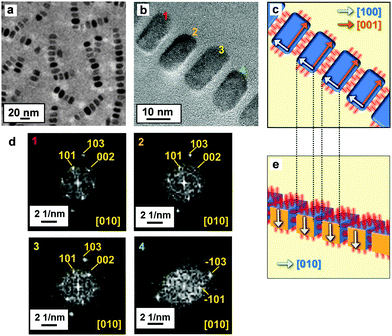
After evaporation of the hydrophobic dispersion medium, we observed the 1D alignment of several Mn3O4 nanocuboids on a collodion film by transmission electron microscopy (TEM) (Fig. 2a). The average number of nanoblocks in the 1D chains was 5.6 ± 3.5. The inter-particle distance (3.0 ± 0.22 nm) is close to twice the molecular length of oleic acid (1.7 nm) (Fig. 2b and c). This suggests that the nanoblocks were covered with the organic molecules that were slightly tilted. Anisotropic Mn3O4 nanoblocks aligned along the a axis (a-axis-oriented 1D alignment) were confirmed by the FFT patterns of lattice fringes obtained from the four adjacent nanocuboids (Fig. 2c and d). Moreover, the nanocrystals in the a-axis-oriented 1D arrays were shown to be highly aligned, like a single crystal, because the crystal zone axes of all nanoblocks corresponded to [010] (Fig. 2e). The deviation of crystallographic orientation was estimated to be ∼2° from FFT patterns of adjacent nanoblocks. Mn3O4 cuboids tend to be aligned in the 〈100〉 direction with side-by-side attachment of rectangular {100} faces. The hydrophobic nature of the surface resulted in the stable dispersion of nanocuboids in a mixture of toluene and hexane. The a-axis-oriented 1D alignment was deduced to be formed through the side-by-side (a face-by-a face) attachment of lateral capillary forces at the air–liquid interface (Fig. 1, Step 2a). Because the surface areas of the rectangular faces are larger than those of the square faces, the rectangular side contacts more effectively decrease the total surface energy of the assemblies than the square side contacts.37
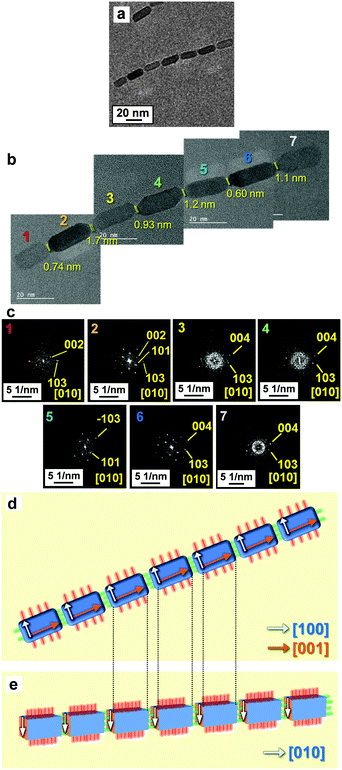
The direction of the alignment was controlled in the same medium by modifying the specific faces of the nanoblocks with organic molecules (Fig. 1, Step 1). The nanoblocks covered with hydrophobic molecules (oleic acid) were dispersed in an aqueous solution of hexamethylenediamine with a 30 min ultrasonication treatment. After the treatment, the end faces (c faces) of the anisotropic Mn3O4 blocks were hydrophilized by replacing oleic acid with hexamethylenediamine. When the resultant nanoblocks were redispersed in the toluene–hexane mixture, linear chains aligned along the c axis (c-axis-oriented 1D alignment) were formed in the liquid medium (Fig. 3a and b). The anisotropic Mn3O4 nanoblocks aligned in the c direction were confirmed by FFT patterns of lattice fringes obtained from the seven adjacent nanocuboids (Fig. 3c and d). Moreover, the nanocrystals in the c-axis-oriented 1D arrays were shown to be highly oriented alignments because the crystal zone axes of all the nanoblocks corresponded to [010] (Fig. 3e). The deviation of crystallographic orientation was estimated to be ∼5° from FFT patterns of the adjacent nanoblocks. The average number of nanoblocks in the c-axis-oriented 1D chains was 3.6 ± 1.7. Because the nanocuboids were spatially separated (inter-particle distance: 1.1 ± 0.35 nm), their surfaces were covered by organic molecules. The thickness of the adsorbed molecular layers, which was smaller than that of the oleic acid molecule (molecular length: 1.7 nm), suggests that oleic acid adsorbed on the c faces was replaced by hexamethylenediamine (molecular length: 0.86 nm) (Fig. 3b). Oleic acid molecules adsorbed onto the surfaces of the nanoblocks are exchanged for hexamethylenediamine by ultrasonication because hydrophobic surfaces are unfavorable in an aqueous medium. Because the surface energy of the a faces is higher than that of the c faces, the detachment of oleic acid molecules from the a face is unfavorable.40 Hydrophobic molecules covering the c faces are preferentially replaced by hexamethylenediamine molecules. Thus, surface-modified nanoblocks with hydrophobic a faces and hydrophilic c faces are obtained. The c-axis-oriented 1D alignment was produced by end-to-end attachment because attaching hydrophilic c faces is favorable in the hydrophobic medium (Fig. 1, Step 2b).
In summary, we fabricated direction-selective 1D architectures consisting of anisotropic nanoblocks through a 2-step process with molecularly mediated manipulation based on the interactions between adsorbed molecules on the block surfaces and a dispersion medium. Nanocuboids were selectively aligned in a hydrophobic medium by modifying the specific faces of the cuboids. Hydrophobic nanoblocks covered with oleic acid molecules are well dispersed in a hydrophobic medium. 1D arrays elongated in the a direction of the tetragonal crystal were produced through side-by-side (a face-by-a face) attachment of the highly dispersed nanocuboids at the air–liquid interface. Surface-modified nanoblocks with hydrophobic a faces and hydrophilic c faces were prepared through selective surface modification of the hydrophobic nanoblocks. Another 1D array aligned in the c direction of the tetragonal crystal was obtained by the end-to-end (c face-to-c face) attachment in a hydrophobic medium by attaching the hydrophilic c faces of the nanocuboids. Faceted cuboids with shape and crystallographic anisotropy are useful building blocks for the fabrication of various elaborate architectures with uniform crystallographic directions. Molecularly mediated manipulation of the building blocks is regarded as a novel direction-controlled accumulation technique for designing a wide variety of functional nanomaterials.
This work was partially supported by the Kato foundation for Promotion of Science, the Advanced Low Carbon Technology Research and Development Program (ALCA) from Japan Science and Technology Agency (JST), a Grant-in-Aid for Challenging Exploratory Research (Grant 15K14129), and a Grant-in-Aid for Scientific Research (Grant 22107010) on Innovative Areas of “Fusion Materials: Creative Development of Materials and Exploration of Their Function through Molecular Control” (Area no. 2206) from the Ministry of Education, Culture, Sports, Science and Technology.
Notes and references
- T. P. Bigioni, X.-M. Lin, T. T. Nguyen, E. I. Corwin, T. A. Witten and H. M. Jaeger, Nat. Mater., 2006, 5, 265 CrossRef CAS PubMed
.
- W. Lu, Q. Liu, Z. Sun, J. He, C. Ezeolu and J. Fang, J. Am. Chem. Soc., 2008, 130, 6983 CrossRef CAS PubMed
.
- T. Yu, J. Moon, J. Park, Y. I. Park, H. B. Na, B. H. Kim, I. C. Song, W. K. Moon and T. Hyeon, Chem. Mater., 2009, 21, 2272 CrossRef CAS
.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS PubMed
.
- A. Martin, C. Schopf, A. Pescaglini, J. J. Wang and D. Iacopino, Langmuir, 2014, 30, 10206 CrossRef CAS PubMed
.
- D. Nykypanchuk, M. M. Maye, D. van der Lelie and O. Gang, Nature, 2008, 451, 549 CrossRef CAS PubMed
.
- M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393 CrossRef CAS
.
- A. Singh, R. D. Gunning, A. Sanyal and K. M. Ryan, Chem. Commun., 2010, 46, 7193 RSC
.
- K. An, N. Lee, J. Park, S. C. Kim, Y. Hwang, J.-G. Park, J.-Y. Kim, J.-H. Park, M. J. Han, J. Yu and T. Hyeon, J. Am. Chem. Soc., 2006, 128, 9753 CrossRef CAS PubMed
.
- S.-Y. Zhang, M. D. Regulacio and M.-Y. Han, Chem. Soc. Rev., 2014, 43, 2301 RSC
.
- H. Nakashima, K. Furukawa, Y. Kashimura and K. Torimitsu, Langmuir, 2008, 24, 5654 CrossRef CAS PubMed
.
- D. Kim, W. D. Kim, M. S. Kang, S.-H. Kim and D. C. Lee, Nano Lett., 2015, 15, 714 CrossRef CAS PubMed
.
- J.-Y. Chang, H. Wu, H. Chen, Y.-C. Ling and W. Tan, Chem. Commun., 2005, 1092 RSC
.
- T. Jain, R. Roodbeen, N. E. A. Reeler, T. Vosch, K. J. Jensen, T. Bjørnholm and K. Nørgaard, J. Colloid Interface Sci., 2012, 376, 83 CrossRef CAS PubMed
.
- Z. Nie, D. Fava, E. Kumacheva, S. Zou, G. C. Walker and M. Rubinstein, Nat. Mater., 2007, 6, 609 CrossRef CAS PubMed
.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576 CrossRef PubMed
.
- C. Chen, J. Wang, Z. Ren, G. Qian and Z. Wang, CrystEngComm, 2014, 16, 1681 RSC
.
- C. Schliehe, B. H. Juarez, M. Pelletier, S. Jander, D. Greshnykh, M. Nagel, A. Meyer, S. Foerster, A. Kornowski and C. Klinke,
et al.
, Science, 2010, 329, 550 CrossRef CAS PubMed
.
- F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Cryst. Growth Des., 2010, 10, 4537 CAS
.
- F. Dang, K. Mimura, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Nanoscale, 2012, 4, 1344 RSC
.
- J. Zhang, H. Yang, K. Yang, J. Fang, S. Zou, Z. Luo, H. Wang, I.-T. Bae and D. Y. Jung, Adv. Funct. Mater., 2010, 20, 3727 CrossRef CAS
.
- A. Demortie`re, P. Launois, N. Goubet, P.-A. Albouy and C. Petit, J. Phys. Chem. B, 2008, 112, 14583 CrossRef PubMed
.
- Z. Quan and J. Fang, Nano Today, 2010, 5, 390 CrossRef CAS
.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS PubMed
.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131 CrossRef CAS PubMed
.
- T. Wang, X. Wang, D. LaMontagne, Z. Wang, Z. Wang and Y. C. Cao, J. Am. Chem. Soc., 2012, 134, 18225 CrossRef CAS PubMed
.
- C.-J. Chen, R.-K. Chiang and Y.-R. Jeng, J. Phys. Chem. C, 2011, 115, 18142 CAS
.
- C.-W. Liao, Y.-S. Lin, K. Chanda, Y.-F. Song and M. H. Huang, J. Am. Chem. Soc., 2013, 135, 2684 CrossRef CAS PubMed
.
- Y. Wang, Y. Zheng, C. Z. Huang and Y. Xia, J. Am. Chem. Soc., 2013, 135, 1941 CrossRef CAS PubMed
.
- A. P. LaGrow, B. Ingham, S. Cheong, G. V. M. Williams, C. Dotzler, M. F. Toney, D. A. Jefferson, E. C. Corbos, P. T. Bishop, J. Cookson and R. D. Tilley, J. Am. Chem. Soc., 2012, 134, 855 CrossRef CAS PubMed
.
- K. Mimura, F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Jpn. J. Appl. Phys., 2011, 50, 09NC09 CrossRef
.
- H. Yang, T. Ogawa, D. Hasegawa and M. Takahashi, J. Appl. Phys., 2008, 103, 07D526 Search PubMed
.
- L. Malaquin, T. Kraus, H. Schmid, E. Delamarche and H. Wolf, Langmuir, 2007, 23, 11513 CrossRef CAS PubMed
.
- J.-M. Meijer, F. Hagemans, L. Rossi, D. V. Byelov, S. I. R. Castillo, A. Snigirev, I. Snigireva, A. P. Philipse and A. V. Petukhov, Langmuir, 2012, 28, 7631 CrossRef CAS PubMed
.
- G. Singh, H. Chan, A. Baskin, E. Gelman, N. Repnin, P. Král and R. Klajn, Science, 2014, 345, 1149 CrossRef CAS PubMed
.
- M. Rycenga, J. M. McLellan and Y. Xia, Adv. Mater., 2008, 20, 2416 CrossRef CAS
.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, J. Am. Chem. Soc., 2014, 136, 3716 CrossRef CAS PubMed
.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, Langmuir, 2015, 31, 6197 CrossRef CAS PubMed
.
- Y. Nakagawa, H. Kageyama, R. Matsumoto, Y. Oaki and H. Imai, CrystEngComm, 2015, 17, 7477 RSC
.
- V. Bayer and R. Podloucky, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 165428 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures. See DOI: 10.1039/c5cc10644c |
| This journal is © The Royal Society of Chemistry 2016 |