Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A practical cobalt-catalyzed cross-coupling of benzylic zinc reagents with aryl and heteroaryl bromides or chlorides†
Andreas D.
Benischke
a,
Irina
Knoll
a,
Alice
Rérat
b,
Corinne
Gosmini
b and
Paul
Knochel
*a
aDepartment Chemie und Biochemie, Ludwig-Maximilians-Universität München, Butenandtstr. 5-13, D-81377 München, Germany. E-mail: knoch@cup.uni-muenchen.de; Fax: +49-89-2180-77680; Tel: +49-89-2180-77681
bLaboratoire de Chimie Moléculaire, CNRS, Ecole Polytechnique, Université Paris Saclay, 91128 Palaiseau, France. E-mail: corinnne.gosmini@polytechnique.edu; Fax: +33-1-6933-4440; Tel: +33-1-6933-4412
First published on 20th January 2016
Abstract
A catalytic system consisting of 5 mol% CoCl2 and 10 mol% isoquinoline allows a convenient cross-coupling of benzylic zinc reagents with various aryl and heteroaryl bromides or chlorides leading to polyfunctionalized diaryl- and aryl-heteroaryl-methane derivatives.
Pd-Catalyzed cross-couplings between organozinc reagents and various organic halides constitute a major C–C bond formation methodology (Negishi cross-coupling).1 Due to the high price and toxicity of palladium, related transition metal-catalyzed cross-couplings involving zinc organometallics and Ni-, Fe- or Co-catalysts have been examined.2–4 Furthermore, the use of zinc organometallics is of special synthetic interest due to the high functional group compatibility of zinc reagents.5 Recently, we have reported several preparation methods of benzylic zinc halides and demonstrated that these reagents undergo smooth Negishi cross-couplings.6 Also Bedford reported that benzylic halides undergo useful Fe-catalyzed cross-couplings with arylzinc reagents.7 Gosmini has shown in one-pot procedures that arylzinc reagents generated in situ via a cobalt-catalyzed zinc insertion undergo cross-couplings with benzyl chlorides.8 Interestingly, Ingleson has described a transition metal free cross-coupling between relatively non-functionalized diarylzincs with benzylic bromides and chlorides performed in the absence of coordinating ethereal solvents.9
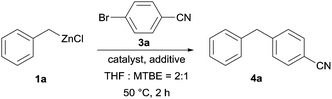
Herein, we report a practical cobalt-catalyzed cross-coupling promoted by 10 mol% of isoquinoline between various benzylic zinc reagents with aryl and heteroaryl bromides or chlorides resulting in the formation of valuable diaryl- and arylheteroaryl-methane derivatives.10 Preliminary control experiments performed with benzylzinc chloride (1a; prepared via the oxidative insertion of magnesium turnings into benzyl chloride (2a) in the presence of LiCl and ZnCl2)11 and 4-bromo-benzonitrile (3a) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF
1 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBE mixture12 (MTBE = methyl tert-butyl ether) show that in the absence of transition catalysts no reaction is observed at 50 °C in 2 h (Table 1, entries 1 and 2). Also, Fe-catalysts such as Fe(acac)3, Fe(acac)2 or FeCl2 were inefficient (Table 1, entries 3–5).13 However, the use of 5 mol% CoBr2, Co(acac)2 and CoCl2 show the formation of the desired cross-coupling product (4a) in 47–76% GC-yield (Table 1, entries 6–8).14
MTBE mixture12 (MTBE = methyl tert-butyl ether) show that in the absence of transition catalysts no reaction is observed at 50 °C in 2 h (Table 1, entries 1 and 2). Also, Fe-catalysts such as Fe(acac)3, Fe(acac)2 or FeCl2 were inefficient (Table 1, entries 3–5).13 However, the use of 5 mol% CoBr2, Co(acac)2 and CoCl2 show the formation of the desired cross-coupling product (4a) in 47–76% GC-yield (Table 1, entries 6–8).14
| Entry | Catalyst (mol%) | Additive (mol%) | Yielda,b |
|---|---|---|---|
| a 1.1 equiv. of benzylzinc chloride (1a) was used. b Determined by GC-analysis with tetradecane as an internal standard. c Isolated yield of pure product. d CoCl2 with a purity of 99.999% was used. | |||
| 1 | None | None | 0 |
| 2 | None | Isoquinoline (10) | 0 |
| 3 | Fe(acac)3 (5) | None | 0 |
| 4 | Fe(acac)2 (5) | None | Traces |
| 5 | FeCl2 (5) | None | Traces |
| 6 | CoBr2 (5) | None | 47 |
| 7 | Co(acac)2 (5) | None | 70 |
| 8 | CoCl2 (5) | None | 76 |
| 9 | CoCl2 (5) | 4-Fluorostyrene (10) | 66 |
| 10 | CoCl2 (5) | TMEDA (10) | 68 |
| 11 | CoCl2 (5) | Isoquinoline (10) | 87 (82)c (72)d |
| 12 | CoCl2 (5) | Isoquinoline (5) | 75 |
| 13 | CoCl2·2LiCl (5) | Isoquinoline (10) | 69 |
| 14 | CoCl2·2LiCl (5) | None | 65 |
Previously reported additives like 4-fluorostyrene,15 TMEDA16 or isoquinoline17 indicate a very positive effect of 10 mol% isoquinoline18 leading to an isolated yield of 82% for 4a (Table 1, entry 11; compared with entries 9 and 10). Decreasing the amount of isoquinoline to 5 mol% reduces somewhat the yield of 4a (Table 1, entry 12). Also, we found that the use of CoCl2·2LiCl19 was not advantageous (Table 1, entries 13 and 14). Additionally, we have examined the influence of the commercial origin of CoCl2 as well as its purity. Thus, CoCl2 having a purity of 99.999% provides under the same conditions (50 °C, 2 h) the diarylmethane 4a in 72% yield (compared to 82%; see Table 1, entry 11).20,21 The addition of MTBE as a cosolvent usually decreases the amount of homo-coupling and therefore enhances the product yield. However, large amounts of MTBE reduce the reaction rate. We found the solvent mixture THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBE 2
MTBE 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to be optimal.22 Concerning the need of isoquinoline as ligand, an extensive screening showed that N-heterocycles behave best, whereas various phosphines did not promote the cross-coupling.23
1 to be optimal.22 Concerning the need of isoquinoline as ligand, an extensive screening showed that N-heterocycles behave best, whereas various phosphines did not promote the cross-coupling.23
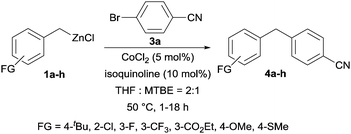
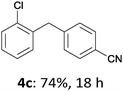
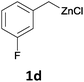
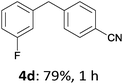
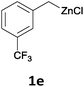
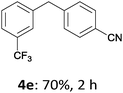
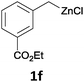
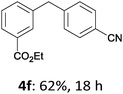
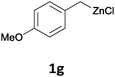
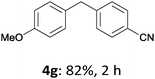
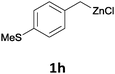
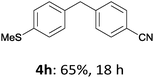
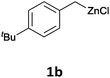
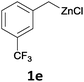
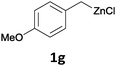
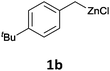
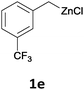
With these optimized conditions in hand, we studied the reaction scope of the cross-coupling between various benzylic zinc chlorides (1a–i) with a broad range of aryl and heteroaryl bromides or chlorides. First, the treatment of benzylic zinc reagents (1a,b) in the presence of 5 mol% CoCl2 and 10 mol% isoquinoline with 4-bromobenzonitrile (3a) at 50 °C within 2 to 4 h is leading to the diarylmethane derivatives 4a,b in 77–82% (Table 2, entries 1 and 2). Furthermore, the cross-coupling of an ortho-substituted benzylzinc chloride (1c) with 3a afforded the desired arene (4c) in 74% yield (Table 2, entry 3). Similarly, the two functionalized benzylic zinc reagents (1d,e) cross-coupled with 3a giving the products 4d,e in 70–79% (Table 2, entries 4 and 5). The ester-substituted benzylzinc chloride (1f) underwent a smooth cross-coupling with 3a leading to the functionalized diaryl-methane 4f in 62% yield (Table 2, entry 6). Additionally, the cross-couplings of the more electron-donating benzylic zinc reagents (1g,h) with 4-bromo-benzonitrile (3a) furnished the arenes 4g,h in 65–82% yield (Table 2, entries 7 and 8).
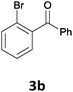
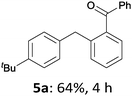
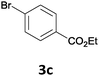
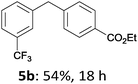
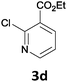
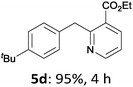
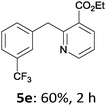
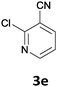
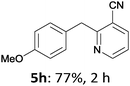
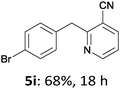
The reaction scope of this cross-coupling proved to be quite broad. Thus, 2-bromo-benzophenone (3b) underwent the cobalt-catalyzed cross-coupling with the benzylzinc chloride (1b) yielding to the corresponding ketone 5a in 64% yield (Table 3, entry 1). Similarly, the coupling of ethyl 4-bromo-benzoate (3c) with the two benzylic zinc reagents (1e,g) led to the functionalized diarylmethane derivatives (5b,c) in 54–70% yield (Table 3, entries 2 and 3). Remarkably, 2-chloropyridines react well with various benzylic zinc reagents (Table 3, entries 4–9). The cross-couplings of the benzylzinc chlorides (1b,e) with ethyl 2-chloronicotinate (3d) proceeded smoothly under these conditions affording the 2,3-disubstituted pyridines (5d,e) in 60–95% yield (Table 3, entries 4 and 5). Also, 3-(ethoxycarbonyl)benzyl-zinc chloride (1f) underwent the coupling with the 2,3-di-substituted pyridine (3d) giving the functionalized aryl-hetero-arylmethane 5f in 68% yield (Table 3, entry 6). Furthermore, the cross-couplings of the benzylic zinc reagents (1d,g,i) with 2-chloro-nicotinonitrile (3e) led to the desired benzylated pyridines (5g–i) in 67–77% yield (Table 3, entries 7–9). Finally, the reaction of 3-fluorobenzylzinc chloride (1d) with ethyl 5-bromofuran-2-carboxylate (3f) afforded within 3 h the 2,5-disubstituted furan (5j) in 60% yield (Table 3, entry 10). The use of aryl bromides bearing electron-donating substituents led to low yields.24
Moreover, such benzylic zinc reagents undergo high yield cross-couplings with various chloro- or bromo-N-heterocycles. Thus, the reaction of 4-methoxybenzylzinc chloride (1g) with 2-bromopyrimidine (3g) and the two substituted pyridines, 2-chloro-5-(trifluoromethyl)pyridine (3h) and 2-chloro-6-fluoro-pyridine (3i), led rapidly (within 2 h) to the functionalized aryl-heteroarylmethanes (6a–c) in 52–83% yield (Scheme 1).
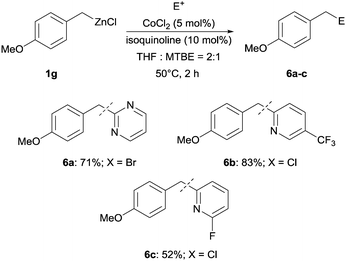 | ||
| Scheme 1 Isoquinoline-promoted cross-coupling of the benzylic zinc reagent 1g with selected N-heterocycles (3g–i). | ||
In summary, we have reported a new practical Co-catalyzed, isoquinoline-promoted cross-coupling of various benzylic zinc chlorides with a range of aryl and heteroaryl bromides and chlorides, producing polyfunctionalized diaryl- or arylhetero-aryl-methane derivatives. This method tolerates a variety of functional groups, such as esters, nitriles or ketones, and proceeds smoothly at 50 °C within 1–18 h. Remarkably, the combination of MTBE (MTBE = methyl tert-butyl ether) as co-solvent and isoquinoline as additive led only to small amounts of homo-coupling. In most cases, shorter reaction times and improved yields could be obtained. Further investigations towards the synthesis and applications of benzylic organo-metallics are underway in our laboratories.
We would like to thank the DFG for financial support. We also thank BASF SE (Ludwigshafen, Germany) and Rockwood Lithium GmbH for the generous gift of chemicals.
Notes and references
- (a) E.-i. Negishi, L. F. Valente and M. Kobayashi, J. Am. Chem. Soc., 1980, 102, 3298 CrossRef CAS; (b) E.-i. Negishi, Acc. Chem. Res., 1982, 15, 340 CrossRef CAS; (c) F. Diederich and P. Stang, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 1998 Search PubMed; (d) A. de Meijere and F. Diederich, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004 Search PubMed.
- For selected Ni-catalyzed Negishi cross-couplings, see: (a) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 14726 CrossRef CAS PubMed; (b) C. Fischer and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 4594 CrossRef CAS PubMed; (c) Y. Tamaru, Modern Organonickel Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed; (d) A. Gavryushin, C. Kofink, G. Manolikakes and P. Knochel, Org. Lett., 2005, 7, 4871 CrossRef CAS PubMed; (e) G. Manolikakes, C. Munoz Hernandez, M. A. Schade, A. Metzger and P. Knochel, J. Org. Chem., 2008, 73, 8422 CrossRef CAS PubMed; (f) M. A. Schade, A. Metzger, S. Hug and P. Knochel, Chem. Commun., 2008, 3046 RSC; (g) L. Melzig, A. Metzger and P. Knochel, J. Org. Chem., 2010, 75, 2131 CrossRef CAS PubMed.
- For selected Fe-catalyzed Negishi cross-couplings, see: (a) A. Fürstner, A. Leitner, M. Méndez and H. Krause, J. Am. Chem. Soc., 2002, 124, 13856 CrossRef; (b) C. Bolm, J. Legros, J. Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed; (c) B. Plietker, Iron-Catalysis in Organic Chemistry: Reactions and Applications, Wiley-VCH, Weinheim, 2008 Search PubMed; (d) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef PubMed.
- For selected Co-catalyzed Negishi cross-couplings, see: (a) J.-M. Bégouin and C. Gosmini, J. Org. Chem., 2009, 74, 3221 CrossRef PubMed; (b) A. Moyeux and G. Cahiez, Chem. Rev., 2010, 110, 1435 CrossRef PubMed; (c) J.-M. Bégouin, M. Rivard and C. Gosmini, Chem. Commun., 2010, 46, 5972 RSC; (d) M. Corpet, X.-Z. Bai and C. Gosmini, Adv. Synth. Catal., 2014, 356, 2937 CrossRef CAS.
- (a) G. Dagousset, C. Francois, T. León, R. Blanc, E. Sansiaume-Dagousset and P. Knochel, Synthesis, 2014, 3133 CAS; (b) T. Klatt, J. T. Markiewicz, C. Saemann and P. Knochel, J. Org. Chem., 2014, 79, 4253 CrossRef CAS PubMed.
- (a) A. Metzger, M. A. Schade and P. Knochel, Org. Lett., 2008, 10, 1107 CrossRef CAS PubMed; (b) A. Metzger, M. A. Schade, G. Manolikakes and P. Knochel, Chem. – Asian J., 2008, 3, 1678 CrossRef CAS PubMed; (c) A. Metzger, F. M. Piller and P. Knochel, Chem. Commun., 2008, 5824 RSC.
- M. Huwe, M. C. Wilkinson and R. B. Bedford, Chem. Commun., 2009, 600 Search PubMed.
- (a) M. Amatore and C. Gosmini, Chem. Commun., 2008, 5019 RSC; (b) J.-M. Bégouin, S. Claudel and C. Gosmini, Synlett, 2009, 3192 Search PubMed.
- J. J. Dunsford, E. R. Clark and M. J. Ingleson, Angew. Chem., Int. Ed., 2015, 54, 5688 CrossRef PubMed.
- (a) W. Hassan, R. Edrada, R. Ebel, V. Wray, A. Berg, R. van Soest, S. Wiryowidagdo and P. Proksch, J. Nat. Prod., 2004, 67, 817 CrossRef PubMed; (b) Z. Jin, Nat. Prod. Rep., 2005, 22, 196 RSC; (c) M. D. Elia and G. A. Molander, J. Org. Chem., 2006, 71, 9198 CrossRef PubMed; (d) N. Kaila, K. Janz, A. Huang, A. Moretto, S. DeBernardo, P. W. Bedard, S. Tam, J. Clerin, J. C. Keith, D. H. H. Tsao, N. Sushkova, G. D. Shaw, R. T. Camphausen, R. G. Schaub and Q. Wang, J. Med. Chem., 2007, 50, 40 CrossRef CAS PubMed.
- A. Metzger, F. M. Piller and P. Knochel, Chem. Commun., 2008, 5824 RSC.
- (a) O. M. Kuzmina, A. K. Steib, D. Flubacher and P. Knochel, Org. Lett., 2012, 14, 4818 CrossRef CAS PubMed; (b) A. K. Steib, O. M. Kuzmina, S. Fernandez, S. Malhotra and P. Knochel, Chem. – Eur. J., 2015, 21, 1961 CrossRef CAS PubMed; (c) O. M. Kuzmina, A. K. Steib, A. Moyeux, G. Cahiez and P. Knochel, Synthesis, 2015, 1696 CAS.
- For recent iron-catalyzed cross-coupling reactions and related reactions, see: (a) C. Bolm, Nat. Chem., 2009, 1, 420 CrossRef CAS PubMed; (b) A. Fürstner, Angew. Chem., Int. Ed., 2009, 48, 1364 CrossRef PubMed; (c) W. M. Czaplik, M. Mayer, J. Cvengros and A. Jacobi von Wangelin, ChemSusChem, 2009, 2, 396 CrossRef CAS PubMed; (d) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS PubMed; (e) C. J. Adams, R. B. Bedford, E. Carter, N. J. Gower, M. F. Haddow, J. N. Harvey, M. Huwe, M. Ángeles Cartes, S. M. Mansell, C. Mendoza, D. M. Murphy, E. Neeve and J. Nunn, J. Am. Chem. Soc., 2012, 134, 10333 CrossRef CAS PubMed; (f) R. B. Bedford, E. Carter, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvey, D. M. Murphy, E. C. Neeve and J. Nunn, Angew. Chem., Int. Ed., 2013, 52, 1285 CrossRef CAS PubMed.
- For recent cobalt-catalyzed cross-coupling reactions and related reactions, see: (a) M. Amatore and C. Gosmini, Chem. – Eur. J., 2010, 16, 5848 CrossRef CAS PubMed; (b) X. Qian, A. Auffrant and C. Gosmini, Angew. Chem., Int. Ed., 2011, 50, 10402 CrossRef CAS PubMed; (c) T. Sawano, K. Ou, T. Nishimura and T. Hayashi, J. Org. Chem., 2013, 78, 8986 CrossRef CAS PubMed; (d) C. E. I. Knappke, S. Grupe, D. Gärtner, M. Corpet, C. Gosmini and A. Jacobi von Wangelin, Chem. – Eur. J., 2014, 20, 6828 CrossRef CAS PubMed; (e) B. Barré, L. Gonnard, R. Campagne, S. Reymond, J. Marin, P. Ciapetti, M. Brellier, A. Guérinot and J. Cossy, Org. Lett., 2014, 16, 6160 CrossRef PubMed; (f) L. Gonnard, A. Guérinot and J. Cossy, Chem. – Eur. J., 2015, 21, 12797 CrossRef CAS PubMed; (g) A. Moyeux, G. Cahiez and J. Cossy, Adv. Synth. Catal., 2015, 357, 1983 CrossRef; (h) N. Sauermann, M. J. Gonzalez and L. Ackermann, Org. Lett., 2015, 17, 5316 CrossRef CAS PubMed; (i) J. Li and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 8551 CrossRef CAS PubMed; (j) M. Moselage, N. Sauermann, S. C. Richter and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 6352 CrossRef CAS PubMed; (k) Y. Cai, X. Qian, A. Rérat, A. Auffrant and C. Gosmini, Adv. Synth. Catal., 2015, 357, 3419 CrossRef CAS.
- (a) A. E. Jensen and P. Knochel, J. Org. Chem., 2002, 67, 79 CrossRef CAS PubMed; (b) T. J. Korn and P. Knochel, Angew. Chem., Int. Ed., 2005, 44, 2947 CrossRef CAS PubMed; (c) S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 2009, 48, 9717 CrossRef CAS PubMed.
- (a) M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS PubMed; (b) M. Nakamura, S. Ito, K. Matsuo and E. Nakamura, Synlett, 2005, 1794 CrossRef CAS; (c) R. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn and C. H. Woodall, Angew. Chem., Int. Ed., 2014, 53, 1804 CrossRef CAS PubMed.
- (a) O. M. Kuzmina, A. K. Steib, J. T. Markiewicz, D. Flubacher and P. Knochel, Angew. Chem., Int. Ed., 2013, 52, 4945 CrossRef CAS PubMed; (b) O. M. Kuzmina, A. K. Steib, S. Fernandez, W. Boudot, J. T. Markiewicz and P. Knochel, Chem. – Eur. J., 2015, 21, 8242 CrossRef CAS PubMed.
- We observed that 2,2′-bipyridine is also a satisfactory ligand for such cross-couplings.
- (a) J. M. Hammann, A. K. Steib and P. Knochel, Org. Lett., 2014, 16, 6500 CrossRef CAS PubMed; (b) J. M. Hammann, D. Haas and P. Knochel, Angew. Chem., Int. Ed., 2015, 54, 4478 CrossRef CAS PubMed.
- (a) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS PubMed; (b) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS PubMed.
- The obtained yield difference using the high purity CoCl2 (99.999%) instead of the commercial CoCl2 (≥97%, Sigma Aldrich) is attributed to the difference of solubility of these two salts. The CoCl2 with the highest purity is significantly less soluble than the 97% pure CoCl2. In fact, this control reaction was performed in only THF.
- For a corresponding solvent screening, see: ESI,† Table S1.
- For an extensive ligand screening, see: ESI,† Table S2.
- Mechanistic studies are underway to explain these phenomena.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc10272c |
| This journal is © The Royal Society of Chemistry 2016 |