 Open Access Article
Open Access ArticleSc3CH@C80: selective 13C enrichment of the central carbon atom†
Katrin
Junghans
,
Marco
Rosenkranz
and
Alexey A.
Popov
*
Leibniz Institute for Solid State and Materials Research, 01069 Dresden, Germany. E-mail: a.popov@ifw-dresden.de
First published on 18th April 2016
Abstract
Sc3CH@C80 is synthesized and characterized by 1H, 13C, and 45Sc NMR. A large negative chemical shift of the proton, −11.73 ppm in the Ih and −8.79 ppm in the D5h C80 cage isomers, is found. 13C satellites in the 1H NMR spectrum enabled indirect determination of the 13C chemical shift for the central carbon at 173 ± 1 ppm. Intensity of the satellites allowed determination of the 13C content for the central carbon atom. This unique possibility is applied to analyze the cluster/cage 13C distribution in mechanistic studies employing either 13CH4 or 13C powder to enrich Sc3CH@C80 with 13C.
The pressure of helium gas is one of the most important parameters affecting the yield of fullerenes in arc-discharge synthesis. Optimization of the atmosphere in the arc-discharge generator (both the pressure and composition) is even more crucial for the synthesis of endohedral metallofullerenes (EMFs) and clusterfullerenes, whose yields are usually much lower than those of empty fullerenes.1 New types of fullerenes or their derivatives can be obtained by introducing different reagents into the arc. Stevenson et al. were the first to show that in the presence of molecular nitrogen, the nitride clusterfullerene Sc3N@C80 can be synthesized in appreciable yield.2 Then, Dunsch et al. demonstrated advantages of the reactive atmosphere method in the synthesis of EMFs: the use of NH3 as a source of nitrogen not only afforded the synthesis of nitride clusterfullerenes, but also suppressed the yield of empty fullerenes.3 The method was adopted for the synthesis of other types of EMF clusterfullerenes, such as sulfides,4 oxides,5 or certain types of carbides.6–9 It was also applied to stabilize unconventional empty fullerene cages via their in situ derivatization by hydrogen10 or chlorine atoms.11 Interestingly, whereas the use of SO2 or CO gases for the synthesis of clusterfullerenes leads to a large amount of empty fullerenes, the hydrogen-containing reagents (NH3, CH4, solid organic compounds12) suppress the yield of empty fullerenes.
Recently we have shown that methane can be advantageous for the synthesis of carbide clusterfullerenes, such as M2TiC@C80 or M2TiC2@C80 (M is Y or a lanthanide).8 It is not clear if methane is barely a source of hydrogen suppressing the empty fullerene formation, or it plays a more specific role by, e.g., supplying the carbon for the endohedral cluster. Answering this question may shed more light on the fullerene formation, but the analysis of the carbon source in the EMF molecule is not straightforward. The use of isotopic substitution would be an obvious way to address this problem, but mass-spectrometry is not able to distinguish the cage and cluster atoms, whereas sensitivity of 13C NMR is not sufficient to allow distribution studies (detection of the central carbon atoms in carbide clusterfullerenes by 13C NMR required 13C enrichment9,13–15). Here we circumvent this problem by studying the Sc3CH@C80, which affords 13C analysis of the central carbon via the 1H NMR signal of the endohedral hydrogen and show that methane plays an active role in the formation of the endohedral cluster.
Sc3CH@C80 was synthesized in two series of experiments, using either pure Sc or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Sc and Ti, as a source of metal. Metals were mixed with graphite powder and packed into the hole-drilled graphite rods, which were then used in the arc-discharge synthesis in the He atmosphere with an addition of several mbar of CH4 (250 mbar total pressure). The main EMF products of the arc discharge synthesis in these conditions are Sc4C2@C80 in pure Sc system, and Sc2TiC@C80 in the mixed-metal Sc/Ti system. Both systems afforded appreciable amounts of Sc3CH@C80, which was further isolated using HPLC (see ESI† for further details of separation).
1 mixture of Sc and Ti, as a source of metal. Metals were mixed with graphite powder and packed into the hole-drilled graphite rods, which were then used in the arc-discharge synthesis in the He atmosphere with an addition of several mbar of CH4 (250 mbar total pressure). The main EMF products of the arc discharge synthesis in these conditions are Sc4C2@C80 in pure Sc system, and Sc2TiC@C80 in the mixed-metal Sc/Ti system. Both systems afforded appreciable amounts of Sc3CH@C80, which was further isolated using HPLC (see ESI† for further details of separation).
Detailed characterization of Sc3CH@C80 in the first report on its synthesis was not possible due to the tiny amounts of the isolated compound.6 In this work, we accomplished characterization of the compound by 45Sc, 13C, and 1H NMR spectroscopy as shown in Fig. 1. The icosahedral cage symmetry of Sc3CH@C80 is proved by 13C NMR spectroscopy (Fig. 1a). Two cage resonances with the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 intensity ratio are observed at 144.06 and 136.78 ppm, which is close to the chemical shifts reported for other clusterfullerenes with the C80-Ih cage2,16–19 (144.57/137.24 ppm in Sc3N@C80,2 144.7/137.8 in Sc4C2@C80,17 144.9/137.7 ppm in Sc3CN@C80,18 or 144.82/137.29 ppm in Sc4O2@C8019). In the 13C-enriched sample, satellite peaks due to coupling of neighboring cage atoms can be seen (Fig. 1a) with the 1JCC coupling constant of 58 Hz, typical for C-sp2 carbon atoms in conjugated π-systems.20 Presumably, the large line-width of the endohedral carbon signal14 did not allow us to detect it in the direct 13C NMR measurements, and the chemical shift of the endohedral carbon was determined from selective decoupling measurements of 1H NMR (see below).
1 intensity ratio are observed at 144.06 and 136.78 ppm, which is close to the chemical shifts reported for other clusterfullerenes with the C80-Ih cage2,16–19 (144.57/137.24 ppm in Sc3N@C80,2 144.7/137.8 in Sc4C2@C80,17 144.9/137.7 ppm in Sc3CN@C80,18 or 144.82/137.29 ppm in Sc4O2@C8019). In the 13C-enriched sample, satellite peaks due to coupling of neighboring cage atoms can be seen (Fig. 1a) with the 1JCC coupling constant of 58 Hz, typical for C-sp2 carbon atoms in conjugated π-systems.20 Presumably, the large line-width of the endohedral carbon signal14 did not allow us to detect it in the direct 13C NMR measurements, and the chemical shift of the endohedral carbon was determined from selective decoupling measurements of 1H NMR (see below).
In 45Sc NMR spectrum, Sc3CH@C80 exhibits a single resonance at 292 ppm (Fig. 1b). This value is 100 ppm low-field with respect to the 45Sc chemical shift in Sc3N@C80 at 191 ppm (Fig. 1b). The proton NMR signal of Sc3CH@C80 was detected at −11.73 ppm (Fig. 1c). Such a high-field value is typical for endohedral protons21–24 (see Table 1). We also detected formation of the second isomer of Sc3CH@C80, presumably with the D5h carbon cage (Fig. S6, ESI†), whose 1H NMR signal is detected at −8.79 ppm.
| EMF | δ(1H) | Ref. | EMF | δ(13C) | Ref. |
|---|---|---|---|---|---|
| a Sc3CH@C80-I and Sc3CH@C80-II denote the major (Ih) and the minor (presumably D5h) isomers. b M = Sc, Y; 2n = 80, 82, 84, 92. | |||||
| Sc3CH@C80-Ia | −11.73 | Sc3CH@C80-Ia | 173 ± 1 | ||
| Sc3CH@C80-IIa | −8.79 | M2C2@C2nb | 220–260 | 13–15 and 25 | |
| H2@C60 | −1.44 | 21 | YCN@C82 | 292.4 | 26 |
| H2O@C60 | −4.81 | 22 | Sc3C2@C80− | 328.3 | 14 |
| H2@C70 | −23.97 | 23 | Lu2TiC@C80 | 340.98 | 9 |
The 1H NMR spectrum of Sc3CH@C80 also exhibits a low intensity doublet due to the proton-bonded 13C (Fig. 1c). The 1JC–H coupling constant is small, 78.5 Hz, which is typical for protons bonded to carbon atoms with highly electropositive substituents. Thus, the measured 1JC–H constant is in line with the large negative charge on the central carbon atom predicted for Sc3CH@C80.27 The possibility to detect 13C satellites in the proton NMR spectrum enables determination of the 13C chemical shift of the central carbon atom via1H NMR measurements with selective 13C decoupling at different 13C irradiation frequencies. The satellites are well visible at 165 or 180 ppm, but disappear completely at 172–175 ppm (Fig. S4, ESI†). Thus, the 13C chemical shift of the central atom in Sc3CH@C80 is determined as 173 ± 1 ppm, which is noticeably downfield than 13C chemical shifts of endohedral carbons in carbide clusterfullerenes (see Table 1).
Sc3CH and Sc3N clusters have the same formal charge (6+) and identical electron count (the (C–H)3− unit is analogous to the nitride ion N3−), and therefore a close similarity of the electronic properties of Sc3CH@C80 and Sc3N@C80 can be expected.6,27 Indeed, both compounds exhibit very similar Vis-NIR absorption spectra (Fig. 2a), with a slight blue shift of the lowest energy band in Sc3CH@C80 (717 nm versus 734 nm in Sc3N@C80). The difference is more distinct in the fluorescence spectra: whereas Sc3CH@C80 exhibits a NIR emission band at 835 nm, the maximum of the fluorescence band of Sc3N@C80 is observed at 910 nm. Crossing points of the absorption and emission bands give the optical gaps of Sc3CH@C80 and Sc3N@C80 as ca. 1.62 and 1.53 eV, respectively. The electrochemical gap of Sc3CH@C80 is also 0.09 V larger than that of Sc3N@C80 (Fig. 2b and Table 2). Both compounds exhibit similar redox behaviour with chemically irreversible first reduction near −1.2 V. DFT calculations show that Sc3CH@C80 and Sc3N@C80 have almost identical spatial distribution of the HOMO and LUMO. The HOMO is essentially a carbon cage orbital, whereas the LUMO has large contribution of Sc atoms (Fig. 2c).
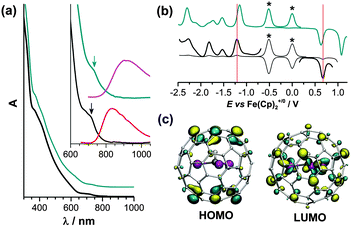 | ||
| Fig. 2 (a) UV-Vis spectra of Sc3CH@C80 (black) and Sc3N@C80 (cyan) in toluene, the inset shows absorption spectra in the range of the lowest energy transitions and luminescence spectra (Sc3CH@C80 – red, Sc3N@C80 – magenta; laser excitation at λex = 405 nm); (b) square wave voltammetry of Sc3CH@C80 (black) and Sc3N@C80 (cyan) in o-dichlorobenzene/TBABF4, asterisks mark Fe(Cp)2 and Fe(Cp*)2 used as internal standards; to guide an eye, the first reduction and oxidation potentials of Sc3CH@C80 are denoted with vertical red lines; (c) HOMO and LUMO of Sc3CH@C80 computed at the PBE/def2-TZVP level.28 | ||
| EMF | O-II | O-I | R-I | R-II | R-III | GapEC |
|---|---|---|---|---|---|---|
| a All potentials are determined by square-wave voltammetry in o-dichlorobenzene/TBABF4 and are referred versus Fe(Cp)2+/0 redox couple; “O” and “R” denote oxidation and reduction, respectively. | ||||||
| Sc3CH@C80 | 0.67 | −1.21 | −1.53/−1.82 | −2.28 | 1.88 | |
| Sc3N@C80 | 1.09 | 0.63 | −1.15 | −1.54/−1.73 | 1.79 | |
Sc3CH@C80 offers a unique possibility to study the role of methane in the carbide clusterfullerene formation using 13C-enrichment. The isotopic distribution of the central carbon atom can be determined by 1H NMR from the intensity of the 13C satellites, whereas the net isotopic distribution in the whole molecule (dominated by that of the carbon cage) can be deduced from the mass-spectra. We synthesized 13C-enriched Sc3CH@C80 by applying either (i) 13CH4 or (ii) 13C powder. To distinguish the two series, they will be denoted as “13CH4/C” and “CH4/13C”, respectively. The amount of 13C powder in the CH4/13C series was adjusted to keep the same amount of 13C in the generator as in the 13CH4/C series. Fig. 3 compares 1H NMR and mass-spectra of the Sc3CH@C80 sample synthesized with the natural-abundant CH4/C to the two types of 13C-enriched samples, whereas estimated 13C content is summarized in Table 3. In the CH4/13C syntheses, the isotopic composition for the central atom and the whole molecule are both equal 5–6% within the uncertainty limits of the NMR measurements (ca. 1%). Thus, the carbon originating from the powder is equally distributed between the cage and the central atom; similar conclusion was achieved by Dorn et al. in their mass-spectrometric study of the empty fullerenes and Y–carbide clusterfullerenes.15
Substantially different results are obtained in the 13CH4/C syntheses: the 13C enrichment of the carbon cage is only 1.6 ± 0.1%, whereas the 13C content for the central atoms is much higher, 7.6 ± 1.5%. Thus, despite the rather large error bars in the NMR measurement (caused by the limited sample amount), selective 13C enrichment of the central carbon atom by 13CH4 is beyond any doubt. The 13CH4/C syntheses thus provide rich information on the Sc3CH@C80 formation process.
The volume inside the generator can be schematically divided into three zones:
(1) The “hot” zone near the center of the arc, where the temperature is up to several thousand K,29 and majority of chemical bonds (including C–H) are broken. Only the most stable species (such as C2 dimers) can survive.
(2) The periphery of the hot zone, where the carbon vapor cools down by the adiabatic expansion and interaction with helium atoms, resulting in a self-assembly of fullerenes and other carbonaceous structures. This intermediate zone is hot enough to provide sufficient energy for the rearrangement of the building carbon networks, but the temperature is not high enough for their atomization.
(3) The “cold” zone, where the fullerenes can only anneal (e.g., structural defects can be healed), but substantial structural rearrangements are already not possible.
We propose that all CH4 molecules entering the hot zone are completely atomized, and therefore the carbon atoms from methane can serve as a source of carbon for the fullerene cage. Since the hot zone occupies only a small volume, whereas methane is distributed over the whole generator chamber, only a small fraction of methane present in the system passes through the hot zone. Thus, it is not surprising that the content of methane-originating carbon atoms in the fullerene cage is not exceeding 0.5%, whereas the main sources of carbon for both the fullerene cage and the endohedral cluster are the graphite rods and graphite powder packed into the rods.
When the C/H vapor leaves the hot zone, the CH bonds can be formed again. Note that from the 0.5% contribution of the methane as a source of carbon for the cage, the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio in the hot zone is tentatively estimated as 50
H ratio in the hot zone is tentatively estimated as 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This ratio is sufficient for a dramatic suppression of the empty fullerene formation. The 13C/12C distribution in the newly formed CH bonds can be considered roughly equal the 13C content in the carbon cage (1.6% in the 13CH4/C syntheses). The fact that the 13C content for the central carbon atom in Sc3CH@C80 obtained in the 13CH4 syntheses is several times higher than for the fullerene cage means that methane is also chemically active in the “intermediate” zone. Although CH4 molecules are not completely atomized here, they can exchange protons with other carbon structures or react with Sc atoms to substitute protons. However, some 13CH fragments remain intact (else the isotopic distribution for the cage and the cluster would be equalized) and take part in the endohedral fullerene formation. The fraction of such “native” 13CH units in Sc3CH@C80 is the difference between the 13C content in the cluster and in the cage, and can be roughly estimated as 6%.
1. This ratio is sufficient for a dramatic suppression of the empty fullerene formation. The 13C/12C distribution in the newly formed CH bonds can be considered roughly equal the 13C content in the carbon cage (1.6% in the 13CH4/C syntheses). The fact that the 13C content for the central carbon atom in Sc3CH@C80 obtained in the 13CH4 syntheses is several times higher than for the fullerene cage means that methane is also chemically active in the “intermediate” zone. Although CH4 molecules are not completely atomized here, they can exchange protons with other carbon structures or react with Sc atoms to substitute protons. However, some 13CH fragments remain intact (else the isotopic distribution for the cage and the cluster would be equalized) and take part in the endohedral fullerene formation. The fraction of such “native” 13CH units in Sc3CH@C80 is the difference between the 13C content in the cluster and in the cage, and can be roughly estimated as 6%.
In this work we reported on the synthesis and spectroscopic characterization of Sc3CH@C80. Its 13C, 45Sc, and 1H NMR spectra are reported for the first time and fully establish the molecular structure of this clusterfullerene. Electronic properties of Sc3CH@C80 are similar to those of its close analog, nitride clusterfullerene Sc3N@C80. Yet, absorption and fluorescence spectroscopy as well as electrochemical study show that the bandgap of Sc3CH@C80 is higher by 0.09 eV. Most importantly, a unique possibility to determine 13C composition of the central atom in the cluster by 1H NMR enables an analysis of the role of methane in the clusterfullerene formation. A series of 13C enrichment with either 13CH4 or 13C powder showed that the use of 13CH4 in the synthesis of Sc3CH@C80 allows selective enrichment of the central carbon atom with 13C.
The authors acknowledge funding by DFG (grant PO 1602/1-2) and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no 648295 “GraM3”). Authors thank Ulrike Nitzsche for technical assistance with computational resources in IFW Dresden.
Notes and references
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989 CrossRef CAS PubMed; X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723 RSC; A. Rodriguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551 RSC.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 401, 55 CrossRef CAS.
- L. Dunsch, M. Krause, J. Noack and P. Georgi, J. Phys. Chem. Solids, 2004, 65, 309 CrossRef CAS; L. Dunsch, P. Georgi, M. Krause and C. R. Wang, Synth. Met., 2003, 135, 761 Search PubMed.
- N. Chen, M. N. Chaur, C. Moore, J. R. Pinzon, R. Valencia, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Commun., 2010, 46, 4818 RSC; N. Chen, M. Mulet-Gas, Y.-Y. Li, R. E. Stene, C. W. Atherton, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Sci., 2013, 4, 180 RSC; N. Chen, C. M. Beavers, M. Mulet-Gas, A. Rodriguez-Fortea, E. J. Munoz, Y.-Y. Li, M. M. Olmstead, A. L. Balch, J. M. Poblet and L. Echegoyen, J. Am. Chem. Soc., 2012, 134, 7851 CrossRef CAS PubMed; F.-F. Li, N. Chen, M. Mulet-Gas, V. Triana, J. Murillo, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Chem. Sci., 2013, 4, 3404 RSC.
- T. Yang, Y. Hao, L. Abella, Q. Tang, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Chem. – Eur. J., 2015, 21, 11110 CrossRef CAS PubMed; M. Zhang, Y. Hao, X. Li, L. Feng, T. Yang, Y. Wan, N. Chen, Z. Slanina, F. Uhlik and H. Cong, J. Phys. Chem. C, 2014, 118, 28883 Search PubMed; Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Inorg. Chem., 2015, 54, 9845 CrossRef PubMed.
- M. Krause, F. Ziegs, A. A. Popov and L. Dunsch, ChemPhysChem, 2007, 8, 537 CrossRef CAS PubMed.
- A. L. Svitova, K. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. Olmstead, A. Balch, L. Dunsch and A. A. Popov, Nat. Commun., 2014, 5, 3568 Search PubMed; Y. Feng, T. Wang, J. Wu, Z. Zhang, L. Jiang, H. Han and C. Wang, Chem. Commun., 2014, 50, 12166 RSC.
- Q. Deng, K. Junghans and A. A. Popov, Theor. Chem. Acc., 2015, 134, 10 CrossRef.
- K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerström, T. Greber, B. Büchner and A. A. Popov, Angew. Chem., Int. Ed. Engl., 2015, 54, 13411 CrossRef CAS PubMed.
- C. R. Wang, Z. Q. Shi, L. J. Wan, X. Lu, L. Dunsch, C. Y. Shu, Y. L. Tang and H. Shinohara, J. Am. Chem. Soc., 2006, 128, 6605 CrossRef CAS PubMed.
- Y.-Z. Tan, X. Han, X. Wu, Y.-Y. Meng, F. Zhu, Z.-Z. Qian, Z.-J. Liao, M.-H. Chen, X. Lu, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, J. Am. Chem. Soc., 2008, 130, 15240 CrossRef CAS PubMed; Y. Z. Tan, J. Li, F. Zhu, X. Han, W. S. Jiang, R. B. Huang, Z. P. Zheng, Z. Z. Qian, R. T. Chen, Z. J. Liao, S. Y. Xie, X. Lu and L. S. Zheng, Nat. Chem., 2010, 2, 269 CrossRef PubMed; S. Y. Xie, F. Gao, X. Lu, R. B. Huang, C. R. Wang, X. Zhang, M. L. Liu, S. L. Deng and L. S. Zheng, Science, 2004, 304, 699 CrossRef PubMed; Y.-Z. Tan, R.-T. Chen, Z.-J. Liao, J. Li, F. Zhu, X. Lu, S.-Y. Xie, J. Li, R.-B. Huang and L.-S. Zheng, Nat. Commun., 2011, 2, 420 CrossRef PubMed; Y.-Z. Tan, S.-Y. Xie, R.-B. Huanh and I.-S. Zheng, Nat. Chem., 2009, 1, 450 CrossRef PubMed.
- S. Yang, L. Zhang, W. Zhang and L. Dunsch, Chem. – Eur. J., 2010, 16, 12398 CrossRef CAS PubMed; M. Jiao, W. Zhang, Y. Xu, T. Wei, C. Chen, F. Liu and S. Yang, Chem. – Eur. J., 2012, 18, 2666 CrossRef PubMed; F. Liu, J. Guan, T. Wei, S. Wang, M. Jiao and S. Yang, Inorg. Chem., 2013, 52, 3814 CrossRef PubMed.
- X. Lu, T. Akasaka and S. Nagase, Acc. Chem. Res., 2013, 46, 1627 CrossRef CAS PubMed; H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka and S. Nagase, J. Am. Chem. Soc., 2011, 133, 2382 CrossRef PubMed; J. Zhang, T. Fuhrer, W. Fu, J. Ge, D. W. Bearden, J. L. Dallas, J. C. Duchamp, K. L. Walker, H. Champion, H. F. Azurmendi, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2012, 134, 8487 CrossRef PubMed.
- Y. Yamazaki, K. Nakajima, T. Wakahara, T. Tsuchiya, M. O. Ishitsuka, Y. Maeda, T. Akasaka, M. Waelchli, N. Mizorogi and H. Nagase, Angew. Chem., Int. Ed. Engl., 2008, 47, 7905 CrossRef CAS PubMed.
- J. Zhang, F. L. Bowles, D. W. Bearden, W. K. Ray, T. Fuhrer, Y. Ye, C. Dixon, K. Harich, R. F. Helm, M. M. Olmstead, A. L. Balch and H. C. Dorn, Nat. Chem., 2013, 5, 880 CrossRef CAS PubMed.
- T. Wang and C. Wang, Acc. Chem. Res., 2014, 47, 450 CrossRef CAS PubMed.
- T.-S. Wang, N. Chen, J.-F. Xiang, B. Li, J.-Y. Wu, W. Xu, L. Jiang, K. Tan, C.-Y. Shu, X. Lu and C.-R. Wang, J. Am. Chem. Soc., 2009, 131, 16646 CrossRef CAS PubMed.
- T.-S. Wang, L. Feng, J.-Y. Wu, W. Xu, J.-F. Xiang, K. Tan, Y.-H. Ma, J.-P. Zheng, L. Jiang, X. Lu, C.-Y. Shu and C.-R. Wang, J. Am. Chem. Soc., 2010, 132, 16362 CrossRef CAS PubMed.
- A. A. Popov, N. Chen, J. R. Pinzón, S. Stevenson, L. A. Echegoyen and L. Dunsch, J. Am. Chem. Soc., 2012, 134, 19607 CrossRef CAS PubMed.
- K. Kamieńska-Trela, in Annu. Rep. NMR Spectrosc., ed. G. A. Webb, Academic Press, 1995, pp. 131 Search PubMed.
- K. Komatsu, M. Murata and Y. Murata, Science, 2005, 307, 238 CrossRef CAS PubMed.
- K. Kurotobi and Y. Murata, Science, 2011, 333, 613 CrossRef CAS PubMed.
- M. Murata, S. Maeda, Y. Morinaka, Y. Murata and K. Komatsu, J. Am. Chem. Soc., 2008, 130, 15800 CrossRef CAS PubMed.
- A. Krachmalnicoff, R. Bounds, S. Mamone, M. H. Levitt, M. Carravetta and R. J. Whitby, Chem. Commun., 2015, 51, 4993 RSC; E. E. Maroto, J. Mateos, M. Garcia-Borràs, S. Osuna, S. Filippone, M. Á. Herranz, Y. Murata, M. Solà and N. Martín, J. Am. Chem. Soc., 2015, 137, 1190 CrossRef CAS PubMed.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2011, 133, 19553 CrossRef CAS PubMed.
- S. Yang, C. Chen, F. Liu, Y. Xie, F. Li, M. Jiao, M. Suzuki, T. Wei, S. Wang, Z. Chen, X. Lu and T. Akasaka, Sci. Rep., 2013, 3, 1487 Search PubMed.
- A. A. Popov and L. Dunsch, Chem. – Eur. J., 2009, 15, 9707 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed; F. Neese, WIREs Comput. Mol. Sci., 2012, 2, 73 CrossRef; W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 CrossRef PubMed.
- H. Lange, K. Saidane, M. Razafinimanana and A. Gleizes, J. Phys. D: Appl. Phys., 1999, 32, 1024 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details, HPLC separation, FTIR and NMR spectra. See DOI: 10.1039/c5cc10025a |
| This journal is © The Royal Society of Chemistry 2016 |


