Open Access Article
Open Access ArticleTetranitratoethane†
Dennis
Fischer
,
Thomas M.
Klapötke
* and
Jörg
Stierstorfer
Ludwig Maximilian University Munich, Department of Chemistry, Butenandtstr. 5-13, 81377 München, Germany. E-mail: tmk@cup.uni-muenchen.de; Web: http://www.hedm.cup.uni-muenchen.de Fax: +49 (0) 89 2180 77492
First published on 13th November 2015
Abstract
Tetranitratoethane (C2H2N4O12), which has an oxygen content of 70.1% was synthesized by nitration of monomeric glyoxal using N2O5 and purified by sublimation. Single crystals could be grown from CH2Cl2/pentane and were used to determine the structure by X-ray diffraction. Several energetic parameters and values were also established.
In the continuous worldwide quest for new oxidizers in order to replace ammonium perchlorate due to its toxicity for humans’ thyroids a few derivatives with a sufficiently high oxygen balance were published during the last years. A few of them are displayed in Fig. 1: (A) tetranitroacetimidic acid,1 (B) nitryl cyanide,2 (C) trinitramine3 and fluorodinitramine,4 and (D) 2,2,2-trinitroethyl nitrocarbamate.5
 | ||
| Fig. 1 Structural formula of tetranitroacetimidic acid (A), nitryl cyanide (B), trinitramine and fluoronitramine (C) as well as 2,2,2-trinitroethyl nitrocarbamate (D). | ||
In general an oxidizer is a material with a positive oxygen balance Ω, having the ability to form additional O2 besides H2O, N2, CO/CO2 during its combustion. The absolute oxygen balance Ω, is the ratio between the amount of active available oxygen divided by the overall mass of oxidizer material. Ω is usually given in % w/w and can be calculated assuming different combustion products e.g. CO2 or CO (see footnote Table 1). In this contribution we report on tetranitratoethane, a new solid state oxidizer, which has a higher oxygen content than prominent solid examples such as ADN (ammonium dinitramide), KClO4, NH4ClO4, tetranitroacetimidic acid and even tetranitromethane. Compound 1 is a geminal dinitrato alkane. While nitrate esters such as nitroglycerin (NG) and pentaerythritoltetranitrate (PETN) (Fig. 2A and B) are well known only very few examples of geminal C-nitrato compounds are known. The simplest one, dinitratomethane (Fig. 2C) is a liquid which is obtained from nitration of 1,3,5-trioxane in a HNO3/H2SO4 mixture.6
| 1 | AP | NG | PETN | |
|---|---|---|---|---|
| a Impact sensitivity according to BAM drophammer (1 of 6). b Friction sensitivity according to BAM friction tester (1 of 6). c Nitrogen and oxygen content. d Oxygen balance toward carbon monoxide (ΩCO = nO − xC − yH/2(1600/FW)) and carbon dioxide (ΩCO2 = nO − 2xC −yH/2(1600/FW)). e Melting and decomposition temperature (DSC, 5 deg min−1). f Density at 298 K (for 1 calculated with: ρX-ray-100K/1.0297). g Heat of formation (calculated using the atomization method and CBS-4M enthalpies). h Energy of formation. i Optimized specific impulse (Cheetah 6.0, shifting equilibrium, 15% w/w HTPB). j Optimized specific impulse (Cheetah 6.0, frozen equilibrium, 15% w/w HTPB). k Optimized amounts of oxidizer and aluminum. | ||||
| Formula | C2H2N4O12 | NH4ClO4 | C3H5N3O9 | C5H8N4O12 |
| FW/g mol−1 | 274.06 | 117.49 | 227.09 | 316.14 |
IS/J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
2 | 20 | 0.2 | 3 |
FS/N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
5 | >360 | >360 | 60 |
N, O%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
20.44, 70.06 | 11.92, 54.47 | 18.5, 63.41 | 17.72, 60.73 |
Ω
CO, ΩCO2/%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
+52.54, +40.87 | 34.04, 34.04 | 3.5, 24.66 | −10.12, 15.18 |
T
m, Tdec/°C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
62, 90 | —, 240 | 13, 185 | 141, 202 |
ρ/g cm−3 (RT)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
1.954 | 1.95 | 1.595* | 1.75* |
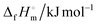 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
−384.6 | −295.8 | −311.3 | −479.7 |
ΔfU°/kJ kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
−1321.9 | −2623.2 | −1278.1 | −1423.3 |
I
sp/s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i i |
272.3 | 263.8 | 263.8 | 256.6 |
I
sp/s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j j |
265.1 | 255.9 | 257.6 | 241.3 |
Ox/Al/% w/w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
65.44/19.56 | 65.85/19.15 | 70.83/14.17 | 69.83/15.62 |
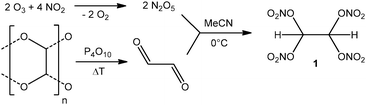
Gemial dinitrate esters form during the nitration of the geminal diol form of aldehydes. They can be also obtained via the addition of N2O5 to the double bond of aldehydes like 1 is obtained from monomeric glyoxal.‡ A stream of monomeric glyoxal was introduced into a solution of N2O5 in acetonitrile at ice bath temperature (Scheme 1). After a few minutes the reaction was poured on ice and crude 1 separated as an oily liquid. The mixture was extracted with CH2Cl2. After removing the solvent crude 1 was obtained as an oil which solidified on standing. The material was purified by sublimation at 70 °C under high vacuum against dry ice. The crystals of the crude material melt at 62 °C before sublimation takes place.
The purified material is stable at room temperature under a dry atmosphere. In air it slowly hydrolyses forming nitric acid and glyoxal again. The hydrolysis however is slow enough to prevent the material from being hydrolyzed in ice water after quenching the reaction. The pure material was slowly crystallized from a dry mixture of CH2Cl2/pentane in a stream of dry nitrogen. Among others one big (ca. 0.4 × 1.0 × 1.0 cm) crystal of 1 was formed over night. The X-ray structure§ reveals the material crystallizing in the orthorhombic space group P21/c with a density of 1.991 g cm−3 at 173 K (Fig. 3). A DSC with a heating rate of 5 °C indicates the material starting to decompose at 90 °C. NMR spectroscopy in CDCl3 revealed a singlet 13C{1H} resonance at 91.3 ppm and a 1H proton resonance at 7.21 ppm.
Compound 1, which has a higher oxygen content and balance than ammonium perchlorate (Table 1) is very sensitive toward friction (5 N) and impact (2 J). In terms of sensitivity it is comparable to nitroglycerin and more sensitive than PETN (Table 1). The heat of formation of −385 kJ mol−1 was calculated using the atomization method based on CBS-4M electronic enthalpies (see ESI†).
The specific impulse from isobaric combustion calculations of three component (oxidizer, aluminum and HTPB) mixtures with optimized oxidizer to aluminum ratio was calculated using the Cheetah 6.0 code. The mixtures using 1 perform slightly higher (8–9 s) than the ammonium perchlorate mixtures and those containing nitroglycerine and PETN (for theoretical comparison). As a general empirical rule, an increase of the value for the specific impulse by 20 s leads to a doubling of the possible payload of a rocket.7
Summarizing all the physicochemical properties of 1, especially the low thermal stability but also the high sensitivities will probably exclude any practical application of 1. Nevertheless 1 is a solid oxidizer carrying one of the highest oxygen contents which were ever synthesized.
Financial support of this work by the Ludwig-Maximilian University of Munich (LMU) and the Office of Naval Research (ONR) is gratefully acknowledged. The authors acknowledge Prof. Dr Karl O. Christe for doing the specific impulse calculations.
Notes and references
- T. T. Vo, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2014, 136, 11934 CrossRef CAS PubMed.
- M. Rahm, G. Belanger-Chabot, R. Haiges and K. O. Christe, Angew. Chem., Int. Ed., 2014, 53, 6893 CrossRef CAS PubMed.
- M. Rahm, S. V. Dvinskikh, I. Furo and T. Brinck, Angew. Chem., Int. Ed., 2011, 50, 1145 CrossRef CAS PubMed.
- K. O. Christe, W. W. Wilson, G. Belanger-Chabot, R. Haiges, J. A. Boatz, M. Rahm, G. K. S. Prakash, T. Saal and M. Hopfinger, Angew. Chem., Int. Ed., 2015, 54, 1316 CrossRef CAS PubMed.
- Q. J. Axthammer, T. M. Klapötke, B. Krumm, R. Moll and S. F. Rest, Z. Anorg. Allg. Chem., 2014, 640, 76 CrossRef CAS.
- G. Travagli, Gazz. Chim. Ital., 1938, 68, 718 CAS.
- T. M. Klapötke, Chemistry of High-Energy Materials, de Gruyter, 3rd edn, Berlin, New York, 2015 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: (1) X-ray parameters, (2) heat of formation calculation, (3) experimental. CCDC 1420413. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc09010e |
‡ 5 g of dehydrated and powdered glyoxal were mixed with 15 g of P4O10 and slowly heated in an oil bath to 200 °C in a 50 mL flask until the material turned black. The green vapors were introduced into an ice cooled solution of 15 g N2O5 in 50 mL CH2Cl2 or CH3CN. Then the reaction was poured on 100 mL ice water and immediately extracted with four times 50 mL CH2Cl2. The organic phase was washed with 1% NaHCO3 until neutral and dried over MgSO4. After carefully (RT) removing the solvent under vacuum the crude material was sublimed at 70 °C under high vacuum against dry ice yielding 7–8 g of a colorless solid. The yield strongly depends on the technique which is used for generating anhydrous glyoxal. Based on monomeric glyoxal the yield is nearly quantitative. DSC (5 °C min−1, °C): 90 °C (dec.); IR (ATR, cm−1): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3000 (w), 2948 (w), 1678 (s), 1664 (s), 1537 (w), 1465 (w), 1342 (w), 1271 (s), 1140 (w), 1048 (m), 989 (s), 821 (m), 771 (vs), 732 (s), 720 (s), 684 (s), 596 (s), 563 (m); Raman (1064 nm, 200 mW, 25 °C, cm−1): = 3000 (w), 2948 (w), 1678 (s), 1664 (s), 1537 (w), 1465 (w), 1342 (w), 1271 (s), 1140 (w), 1048 (m), 989 (s), 821 (m), 771 (vs), 732 (s), 720 (s), 684 (s), 596 (s), 563 (m); Raman (1064 nm, 200 mW, 25 °C, cm−1): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 2997 (54), 2859 (6), 1729 (6), 1693 (50), 1671 (10), 1465 (6), 1356 (45), 1306 (90), 1278 (13), 1148 (47), 1074 (10), 1014 (15), 855 (100), 790 (6), 778 (14), 757 (11), 734 (8), 675 (64), 626 (22), 579 (53); 1H NMR (400 MHz, CDCl3, 25 °C, ppm): δ = 7.21; 13C NMR{1H} (400 MHz, CDCl3, 25 °C, ppm) δ = 91.3; 14N NMR (400 MHz, CDCl3, 25 °C, ppm) δ = −62.3; EA (C2H2N4O12, 174.06): calc.: C 8.77, H 0.74, N 20.44%; found: C 8.97, H 0.83, N 20.19%; BAM drophammer: 2 J (>500 μm); friction tester:<5 N (>500 μm). = 2997 (54), 2859 (6), 1729 (6), 1693 (50), 1671 (10), 1465 (6), 1356 (45), 1306 (90), 1278 (13), 1148 (47), 1074 (10), 1014 (15), 855 (100), 790 (6), 778 (14), 757 (11), 734 (8), 675 (64), 626 (22), 579 (53); 1H NMR (400 MHz, CDCl3, 25 °C, ppm): δ = 7.21; 13C NMR{1H} (400 MHz, CDCl3, 25 °C, ppm) δ = 91.3; 14N NMR (400 MHz, CDCl3, 25 °C, ppm) δ = −62.3; EA (C2H2N4O12, 174.06): calc.: C 8.77, H 0.74, N 20.44%; found: C 8.97, H 0.83, N 20.19%; BAM drophammer: 2 J (>500 μm); friction tester:<5 N (>500 μm). |
| § Selected X-ray parameters: monoclinic, P21/c, a 7.5489(4) Å, b 7.2995(3) Å, c 8.3759(8) Å, γ 97.93(1)°, V 457.12(5), Z 2, ρ 1.991 g cm−3, CCDC 1420413. |
| This journal is © The Royal Society of Chemistry 2016 |