 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rearrangement of {α-P2W15} to {PW6} moieties during the assembly of transition-metal-linked polyoxometalate clusters†
Mercè
Martin-Sabi
,
Ross Stuart
Winter
,
Claire
Lydon
,
Jamie M.
Cameron
,
De-Liang
Long
and
Leroy
Cronin
*
School of Chemistry, WestCHEM, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: lee.cronin@glasgow.ac.uk
First published on 9th November 2015
Abstract
We report the formation of two polyoxotungstates of the general formula [M6(PW6O26)(α-P2W15O56)2(H2O)2]23− (M = CoII or MnII), which contain {PW6} fragments generated from the [P2W15O56]12− precursor, which demonstrates for the first time the transformation of a Dawson lacunae into a Keggin lacunary building block. Solution analysis of the clusters has been conducted via electrospray ionisation mass spectrometry.
Polyoxometalates (POMs) are molecular oxide units generally formed by highly oxidised transition metal atoms such as W6+, Mo5+/6+, etc. that yield in most cases highly charged anionic clusters.1 These clusters have a wide range of different architectures and may possess properties applicable to diverse fields such as catalysis,2 material science,3 nanotechnology,4 magnetism,5 and medicine.6 By incorporating other metals to make transition metal substituted polyoxometalates (TMSPs), the structural diversity and functionality of the anions is increased.7 Many TMSPs are based upon either Keggin8 or Dawson9 fragments, this is due to the wide library of lacunary building blocks available10 for both of these parent architectures. One of the interesting aspects of using lacunary precursors is the transformations that can occur when reacting with transition metals, but this is more commonly seen for Keggin type starting materials.11
Herein we report two structurally analogous compounds that show the previously unseen transformation of the Dawson-type {α-P2W15} precursor to a Keggin-type {PW6} fragment. Both the cobalt and manganese containing TMSPs are obtained using a synthesis adapted from previously reported methodology.12 The solution behaviour of the cluster is performed using ESI-MS and structural characterization is performed by XRD crystallography.
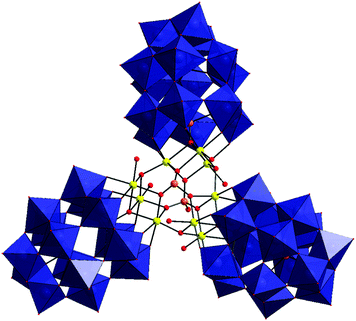
Compounds 1 and 2, Na9Li14[Co6(PW6O26)(α-P2W15O56)2(H2O)2]·55H2O and Na11Li12[Mn6(PW6O26)(α-P2W15O56)2(H2O)2]·58H2O are analogues, possessing a V-shaped sandwich structure (Fig. 1). The general formula for the anion is [M6(PW6O26)(α-P2W15O56)2(H2O)2]23− (M = Co2+(1a), Mn2+(2a)). These anionic units consist of two {α-P2W15} units each connected by three transition metal atoms to a central {PW6} species, creating a bent architecture. Both clusters belong to the C2v point group and crystallise in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. For these compounds the bending angle as defined between the phosphorus atoms of the {α-P2W15} and the phosphorus of the central {PW6} unit, is 145° for 1a and 141° for 2a. The P–O bond lengths of the central {PW6} unit are between 1.51–1.55 Å for 1a and 1.54–1.58 Å for 2a. The M–O bond lengths are between 2.00–2.21 Å for 1a (M = Co) and 2.10–2.29 Å for 2a (M = Mn). The terminal M–O bond lengths are 2.11–2.13 Å for 1a (M = Co) and 2.17–2.19 Å for 2a (M = Mn) which is indicative that these oxygen atoms are water molecules. This is confirmed by bond valence sum (BVS) calculations, which also confirm that all the Co and Mn atoms are in the 2+ oxidation state.
space group. For these compounds the bending angle as defined between the phosphorus atoms of the {α-P2W15} and the phosphorus of the central {PW6} unit, is 145° for 1a and 141° for 2a. The P–O bond lengths of the central {PW6} unit are between 1.51–1.55 Å for 1a and 1.54–1.58 Å for 2a. The M–O bond lengths are between 2.00–2.21 Å for 1a (M = Co) and 2.10–2.29 Å for 2a (M = Mn). The terminal M–O bond lengths are 2.11–2.13 Å for 1a (M = Co) and 2.17–2.19 Å for 2a (M = Mn) which is indicative that these oxygen atoms are water molecules. This is confirmed by bond valence sum (BVS) calculations, which also confirm that all the Co and Mn atoms are in the 2+ oxidation state.
Compounds 1 and 2 were synthesised using Na12[α-P2W15O56]·24H2O as the lacunary precursor in the presence of the appropriate transition metal ions, lithium chloride and sodium phosphate as a slurry in a minimal amount of solvent in the pH range 6–7. If the pH value is raised to values higher than pH 7.5 then Na11Li14H2[M9(H2O)6(α-P2W15O56)3]·65H2O can crystallise.13 For manganese, this trimer (compound 3, see Fig. 3) was observed only a few times in low yield and full characterisation has not been possible due to lack of material. We can thus see that a small pH change can lead to a different set of structures and we deduce that pH control is the most important factor for the synthesis of these compounds. It is worth noting that in all these reactions, the [M4(P2W15)2] sandwich14 was also obtained as a by-product, which is expected given that {P2W15} is not known to reorganise easily.
The nature of the synthetic strategy used makes it difficult to follow the reaction using analytical methods that would give an insight into the mechanism. This is because the reaction requires such a large excess of Na12[α-P2W15O56]·24H2O that any in situ analysis was unfeasible due to the super-saturation and turbidity of the reaction mixture.
There are a few examples in the literature that report banana or V-shaped sandwiches, but all of these architectures are based on Keggin building blocks.15 All of them have the common {XW6} unit that hinges both parts of the cluster. An iron substituted species that was published by Mialane et al. is a single molecule magnet.16 Compounds 1 and 2 are special banana TMSPs because there is no known TMSP structure that contains both an {α-P2W15} and a {PW6} unit. There are rare examples of mixed Dawson/Keggin species,17 however we report the first example in which the distinct Keggin and Dawson lacunae have arisen via the transformation of a single lacunary parent. The transformation from {α-P2W15} to {PW6} is unexpected as the {α-P2W15} Dawson species contains no {PW6} unit of matching connectivity in its structure and it is difficult to postulate how this transformation could occur. A possible mechanism would be the initial decomposition of {α-P2W15} to give free tungstate and then it would assemble in two {W3} triads that would react with the {PO4} template generating a {PW6} unit.18
In order to investigate the stability of compounds 1 and 2 in solution, electrospray ionisation mass spectrometry (ESI-MS) analysis was conducted. Ionisation for compound 1 was successful and a full spectrum of assignable envelopes was obtained. The ESI-MS spectrum (see Fig. 2, top) shows three envelopes that correspond to the intact cluster. These can be found at the charge values of −6, −7, and −8 at m/z of 1595.5 {Na3Li2H12[Co6(PW6O26)(α-P2W15O56)2(H2O)2]·6H2O}, 1359.8 {Na3Li2H11[Co6(PW6O26)(α-P2W15O56)2(H2O)2]·3H2O} and 1186.8 {Na2Li2H11[Co6(PW6O26)(α-P2W15O56)2(H2O)2]·3H2O}, respectively. A fragment {Co3P2W15} species is found at a lower m/z value of 845.4, at a charge of −5 which corresponds to {Na1Li1H5[Co3P2W15O59(H2O)3]·11H2O} (see ESI,† Section 5).
Ionisation was also successful for compound 2 and a full spectrum was obtained (see Fig. 2, bottom). In this case there are three envelopes that correspond to the intact cluster. These are found at charge values of −6, −7, and −8 at m/z of 1599.9 {Na5Li12[Mn6(PW6O26)(P2W15O56)2(H2O)2]·3H2O}, 1363.5 {Na2Li11H3[Mn6(PW6O26)(P2W15O56)2(H2O)2]·4H2O} and 1187.2 {Li14H[Mn6(PW6O26)(P2W15O56)2(H2O)2]·3H2O}. Other peaks at lower m/z values can also be found and they correspond to fragment {Mn3P2W15} species. These can be found at the charge values of −5, −5 and −4 at m/z of 817.0 {Li4H3[Mn3P2W15O59(H2O)3]·4H2O}, 846.2 {Na1Li1H5[Mn3P2W15O59(H2O)3]·12H2O} and 1025.5 {Na1Li3H4[Mn3P2W15O59(H2O)3]·4H2O} (see ESI,† Section 5).
Compound 3 is a trimeric structure that consists of a core of nine manganese centres templated by two phosphate units (see Fig. 3). This core is encased by three {α-P2W15} units. The full formula of this compound cannot be given due to a lack of reproducibility and very low yields resulting in a full characterisation not being possible. The formula derived solely from crystallographic analysis is Na5Li20[W45Mn9P8O174(OH)5(H2O)6]·60H2O. BVS calculations show that the oxidation state for all the manganese atoms is 2+. The bond lengths of the central phosphate templates are between 1.47–1.62 Å for the bridging oxygens and 1.70–1.71 Å for the terminal oxygen atoms, indicative that the terminal oxygen on each central phosphate is a water molecule. The bridging Mn–O distances are between 2.04–2.33 Å and the terminal Mn–O distances are between 2.16–2.25 Å, which indicate that each terminal oxygen corresponds to a water molecule.
 | ||
| Fig. 3 Polyhedral and ball-and-stick representation of compound 3 (Mn trimer). Colour scheme: indigo polyhedra: W, yellow spheres: Mn, orange spheres: P and red spheres: O. | ||
Three new compounds containing Dawson fragments have been reported. Two of them also possess the Keggin-type {PW6} building block. This has been generated from {α-P2W15}, an unprecedented transformation which shows that Dawson lacunae may be more versatile than once thought. The {PW6} fragment is useful for controlling the shape of a cluster because the lacunary positions are oriented such that an angle is introduced into the architecture. It would be of interest to see if by modifying our synthesis whether multiple {XW6} fragments could be trapped within a given architecture and what effect this would have. By better understanding the rearrangement that these building blocks undertake in solution, better design and control of the formation of new clusters, with higher nuclearities and predefined shapes can be achieved.
This work was supported by the EPSRC (grants EP/L023652/1; EP/K038885/1; EP/H024107/1; EP/K023004/1; EP/K021966/1; EP/I033459/1; EP/J015156/1), the EU project 318671 MICREAGENTS and the University of Glasgow. We would like to thank Michael Beglan for his contribution toward the flame atomic absorption measurements and Dr Diana Castro Spencer, Dr Jennifer S. Mathieson and Philip J. Robins for ESI-MS work.
References
-
(a)
M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, 1983 Search PubMed
; (b) M. T. Pope and U. Kortz, “Polyoxometalates” in Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley-VCH, Weinheim, 2012 Search PubMed
; (c) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–38 CrossRef
; (d) C. L. Hill, Special issue on polyoxometalates, Chem. Rev., 1998, 98, 1–2.
- C. L. Hill, G.-S. Kim, C. M. Prosser-Mc-Cartha and D. Judd, Mol. Eng., 1993, 3, 263–275 CrossRef CAS
.
- C. Busche, L. Vila-Nadal, J. Yan, H. N. Miras, D.-L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet and L. Cronin, Nature, 2014, 515, 545–549 CrossRef CAS PubMed
.
- C. Fleming, D.-L. Long, N. McMillan, J. Johnson, N. Bovet, V. Dhanak, N. Gadegaard, P. Kögerler, L. Cronin and M. Kadodwala, Nat. Nanotechnol., 2008, 3, 229–233 CrossRef CAS PubMed
.
- H. Imai, T. Akutagawa, F. Kudo, M. Ito, K. Toyoda, S.-I. Noro, L. Cronin and T. Nakamura, J. Am. Chem. Soc., 2009, 131, 13578–13579 CrossRef CAS PubMed
.
- J. T. Rhule, C. L. Hill and D. A. Judd, Chem. Rev., 1998, 98, 327–357 CrossRef CAS PubMed
.
- S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623–7646 RSC
.
- J. F. Keggin, Nature, 1933, 132, 351 CrossRef CAS
.
- B. Dawson, Acta Crystallogr., 1953, 6, 113–126 CrossRef CAS
.
-
R. Contant, W. G. Kemplerer and O. Yaghi, Inorganic Syntheses, John Wiley & Sons, Inc., 2007, vol. 27, p. 108 Search PubMed
.
- B. S. Bassil and U. Kortz, Dalton Trans., 2011, 40, 9649–9661 RSC
.
- T. M. Anderson, X. Zhang, K. I. Hardcastle and C. L. Hill, Inorg. Chem., 2002, 41, 2477–2488 CrossRef CAS PubMed
.
- C. Lydon, M. M. Sabi, M. D. Symes, D.-L. Long, M. Murrie, S. Yoshii, H. Nojiri and L. Cronin, Chem. Commun., 2012, 48, 9819–9821 RSC
.
- R. G. Finke, M. W. Droege and P. J. Domaille, Inorg. Chem., 1987, 26, 3886–3896 CrossRef CAS
.
-
(a) I. M. Mbomekallé, B. Keita, M. Nierlich, U. Kortz, P. Berthet and L. Nadjo, Inorg. Chem., 2003, 42, 5143–5152 CrossRef PubMed
; (b) T. Ruizhan, L. Chen, Y. Liu, B. Liu, G. Xue, H. Hu, F. Fu and J. Wang, Inorg. Chem. Commun., 2010, 13, 98–100 CrossRef
; (c) Z. Zhang, E. Wang, W. Chen and H. Tan, Aust. J. Chem., 2007, 60, 284–290 CrossRef CAS
.
- J.-D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekallé, J. Marrot, F. Sécheresse, E. Riviére, G. Rogez and W. Wernsdorfer, Angew. Chem., Int. Ed., 2009, 48, 3077–3081 CrossRef CAS PubMed
.
-
(a) J. M. Cameron, J. Gao, D.-L. Long and L. Cronin, Inorg. Chem. Front., 2014, 1, 178–185 RSC
; (b) W.-C. Chen, W.-K. Yan, C.-X. Wu, X.-L. Wang, K.-Z. Shao, Z.-M. Su and E.-B. Wang, Cryst. Growth Des., 2014, 14, 5099–5110 CrossRef CAS
.
- L. Vilà-Nadal, S. G. Mitchell, A. Rodríguez-Fortea, H. N. Miras, L. Cronin and J. M. Poblet, Phys. Chem. Chem. Phys., 2011, 13, 20136–20145 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, crystallographic structures of the clusters and tables, ESI-MS for compounds 1 and 2, and UV-Vis. CCDC 1430716–1430718. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc08486e |
| This journal is © The Royal Society of Chemistry 2016 |


