Open Access Article
Open Access ArticleA mesoionic bis(Py-tzNHC) palladium(II) complex catalyses “green” Sonogashira reaction through an unprecedented mechanism†‡
Martin
Gazvoda
,
Miha
Virant
,
Andrej
Pevec
,
Damijana
Urankar
,
Aljoša
Bolje
,
Marijan
Kočevar
and
Janez
Košmrlj
*
Faculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, SI-1000 Ljubljana, Slovenia. E-mail: janez.kosmrlj@fkkt.uni-lj.si
First published on 6th November 2015
Abstract
A novel bis(pyridyl-functionalized 1,2,3-triazol-5-ylidene)-palladium(II) complex [Pd(Py-tzNHC)2]2+ catalyses the copper-, amine-, phosphine-, and additive-free aerobic Sonogashira alkynylation of (hetero)aryl bromides in water as the only reaction solvent. The catalysis proceeds along two connected Pd-cycles with homogeneous bis-carbene Pd0 and PdII species, as demonstrated by electrospray ionization mass spectrometry.
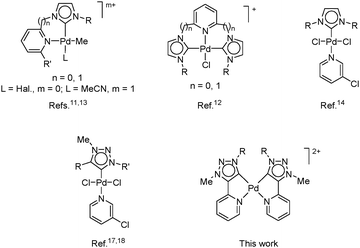
Initiated by the report of Arduengo et al. in 1991 on the first isolation of N-heterocyclic carbene (NHC),1 this class of compounds has become one of the most important ligands in transition-metal catalysis. NHCs have been introduced as ligands in palladium complexes2–9 to support and activate palladium in various cross-coupling reactions, in particular the Heck and Suzuki reactions.10 In this context, pyridine functionalized imidazolin-2-ylidene NHCs as chelating ligands for palladium have been developed (Fig. 1),11–13 followed by Pd-NHCs from a PEPPSI (pyridine-enhanced precatalyst preparation, stabilization, and initiation) series with a further improved stability and activity profile.14 The success of normal NHC ligands is greatly attributed to their superior σ-donating capabilities as compared to phosphines, which is even greater in abnormal NHC counterparts.15,16 Interesting examples are based on the mesoionic 1,2,3-triazol-5-ylidene (tzNHC) structure8 including those of the PEPPSI type reported recently.17,18
Appropriate balancing of the stability of the palladium species is essential in designing better catalysts. We surmised that the bis-bidentate palladium complex of chelating pyridine-functionalized tzNHC featuring a highly stabilizing mesoionic carbenic structure and a donor pyridine substituent should possess unique properties in terms of stability and catalytic activity (Fig. 1). We aimed at developing a palladium catalyst for Sonogashira cross-coupling that would enable copper- and amine-free alkynylation of aryl halogenides that operate in water, in the presence of air, and in the absence of any additive. The Sonogashira reaction19 has witnessed a tremendous success in both academia and industry, being used as the key step in the synthesis of many natural products, bio-active compounds and pharmaceuticals.20–22 It should be noted, however, that protocols allowing the presence of air and employing water as the only reaction solvent are scarce23 and no such example is reported for Pd-NHCs as catalysts.10,15,16,23,24 Herein, we report a highly efficient novel palladium bis(Py-tzNHC) complex (Fig. 1) that catalyses Sonogashira reaction under green reaction conditions, operating through an unprecedented mechanism.
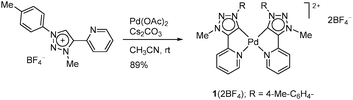
Cationic complex 1(2BF4) was easily prepared in air by a one-step route through direct metalation of the appropriate triazolium cation25 with Pd(OAc)2 in the presence of a weak base, without requiring preactivation with Ag2O (Scheme 1). A water soluble air-stable product was isolated in pure form in 89% yield by a simple workup. The carbene signal in the 13C NMR spectrum of 1 appears at 143.6 ppm, which is indicative of a tzNHC-Pd-complex having pyridine and carbene in the trans position.26 In the 1H NMR spectra a small up-field shift of the PyH-6 resonance upon formation of 1 from the triazolium cation (Δδ ≈ 0.1 ppm; DMSO-d6, DMF-d7, CD3CN) suggested weak interactions between the two pyridine wingtips and palladium, which are essential to stabilize the complex, yet to provide an open coordination site for catalysis to occur (ESI‡). Interestingly, in the solid state, complex 1(2BF4) forms a bi-metallic structure 1′ with a short Pd–Pd intermetallic distance of 3.0232(4) Å (Fig. 2). Upon dissolution 1′ instantly transforms into 1 as evident from 1H NMR and ESI-HRMS analyses.
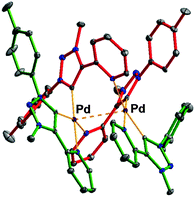 | ||
| Fig. 2 Ortep drawing (30% probability ellipsoids) of cation 1′ (blue = N, gray = C, and violet = Pd) with bidentate (green) and bridging coordination (red). Anions, solvents and hydrogen atoms are omitted for clarity (ESI‡). | ||
Complex 1(2BF4) was evaluated as a precatalyst for the Sonogashira reaction. An initial screening revealed that it effectively cross-coupled acetylenes with aryl iodides and bromides in the presence of air and in water as the only solvent (ESI‡).
To identify the optimal reaction conditions for Sonogashira cross-coupling with 1(2BF4), the effect of the catalyst loading, reaction temperature and base was screened (ESI‡). Excellent results were obtained with 1 mol% of 1(2BF4) at 100–140 °C for 1–4 h, with carbonate (K2CO3 or Cs2CO3) base.
The results of the substrate scope screening are shown in Table 1. In general, 1 mol% of 1(2BF4) effectively catalysed the alkynylation of electron-poor and electron-rich aryl bromides. For coupling of those substrates that are sparingly soluble in hot water, i.e. 4-bromonitrobenzene (2c), the addition of DMF to the reaction mixture proved to be beneficial. Although the highly deactivated 4-methoxybromobenzene (2d) was coupled with 3a in only 36%, m- and p-bromotoluene 2e and 2f reacted quantitatively. Both electron-rich and electron-poor heterocyclic substrates including 2-bromopyridine (2g), 2-bromopyrimidine (2h) and 3-bromothiophene (2i) reacted with acetylenes in good to excellent yields. The general applicability of 1(2BF4) was also confirmed through the selection of electron-rich and deficient acetylenes as coupling partners including 4-ethynylanisole (3b), (triisopropylsilyl)acetylene (3c), 4-ethynyl-α,α,α-trifluorotoluene (3d) and dimethyl ethynyl carbinol (3e), reacting smoothly with excellent yields of 4(i–l). These results clearly demonstrate the robustness and general superior catalytic activity of 1(2BF4) over the monodentate tzNHC palladium complexes.16
| Entry | 2 | 3 | Cond.a | 4 | Conv.b (Yield)c |
|---|---|---|---|---|---|
| a Conditions A: bromide 2 (0.25 mmol), acetylene 3 (0.5 mmol), Cs2CO3 (0.5 mmol), complex 1(2BF4) (0.0025 mmol, 1.0 mol%), water (2 mL), 100 °C in an ACE tube, 1 h. Conditions B: as for conditions A but with K2CO3 (0.5 mmol) as a base, at 140 °C for 4 h. b Conversion determined from at least two consecutive runs by 1H NMR. c Percent yield of the isolated pure product. d DMF/H2O (2/1) as the reaction solvent. | |||||
| 1 | 2a | 3a | Ad |

|
100 |
| 2 | B | 82 (75) | |||
| 3 | 2b | 3a | A |

|
100 |
| 4 | B | 100 (92) | |||
| 5 | 2c | 3a | B |

|
68 (65) |
| 6 | Ad | 100 (91) | |||
| 7 | 2d | 3a | Bd |

|
36 |
| 8 | 2e | 3a | B |

|
100 (95) |
| 9 | 2f | 3a | A |
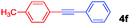
|
60 (58) |
| 10 | Ad | 100 (86) | |||
| 11 | 2g | 3a | B |

|
100 (97) |
| 12 | 2h | 3a | B |

|
66 |
| 13 | 2a | 3b | B |

|
100 (91) |
| 14 | 2f | 3c | B |

|
100 (95) |
| 15 | 2b | 3d | B |
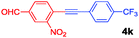
|
100 (89) |
| 16 | 2i | 3e | A |

|
90 (87) |
To get a feel of the potency of the catalyst under typical Sonogashira reaction conditions that are normally applied, we selected the alkynylation of 2a with 3a at 100 °C in DMF and in the presence of DABCO as a base.21 By using 1(2BF4) in 0.1 mol% loading the formation of 4a was quantitative within 1 h. With 0.01 mol%, the transformation was 98% in 8 h (ESI‡).
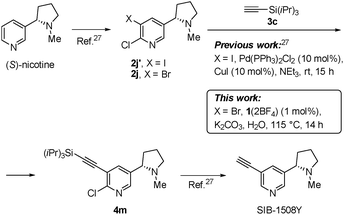
We have been interested in the synthesis of SIB-1508Y (Altinicline), a potential drug for neurodegenerative diseases.22 An expedient five-step preparation of SIB-1508Y with selective halogenation of natural (S)-nicotine to iodide 2j′ and a subsequent “classical” Sonogashira reaction with 3c to the intermediate product 4m has been reported (Scheme 2).27 To demonstrate the robustness of 1(2BF4) and render it practicable, we tested it under green reaction conditions for the synthesis of 4m from bromide 2j, instead of iodide 2j′. Bromide 2j was let to react with 3c in the presence of 1 mol% of 1(2BF4) to afford 4m in 82% yield in optically pure form, without racemization (Scheme 2).
It is known that some Pd-tzNHCs used in the cross-coupling reactions give under very mild conditions palladium nanoparticles as the catalytically active phase.17 In an independent representative experiment by using 1(2BF4) for the cross-coupling of 2a with 3a under the conditions B from Table 1 (vigorous stirring) a large excess of Hg(0) was added (mercury poisoning experiment)17,28,29 into the reaction mixture after 30 min (36% conversion). This addition had no effect on the conversion into 4a, reaching 76% after 4 h; a parallel Hg-free reaction reached 75% over the same period (qNMR assay). A similar observation was made when Hg(0) was added to the reaction mixture at the onset (ESI‡). The thermal stability of complex 1(2BF4) was ascertained in the solid state at 150 °C and in solution (DMF-d7, D2O) at 140 °C (the highest reaction temperatures used herein for the Sonogashira reaction). No decomposition could be detected by qNMR (ESI‡) indicating its remarkable stability over some related PEPPSI-type Pd-tzNHC complexes.17 These results suggest that the catalysis with 1(2BF4) occurs by in situ generated homogeneous catalytically active molecular Pd0 species.
To get an insight into the mechanism of this process, the coupling of 2a with 3a in the presence of Cs2CO3 in DMF was monitored by high-resolution electrospray ionization mass spectrometry (ESI-HRMS).29,30 All peaks from the mass spectra have been identified. As evident from the characteristic isotopic pattern, only mono-palladium bis-carbene cationic species could be found in the spectra. These include ions at m/z 711.1849 (calcd for C37H33N8OPd+ ([A − Br]+): 711.1807), m/z 813.2299 (calcd for C45H39N8OPd+ ([B + H]+): 813.2276), m/z 707.1856 (calcd for C38H33N8Pd+ ([C]+): 707.1858) and m/z 685.0642 (calcd for C30H2879BrN8Pd+ ([D]+): 685.0650) (Scheme 3). Peaks for C and D were the most intensive in the ESI-MS spectra. In contrast to some Pd–NHC complexes,17,29 neither clusters of the type [Pdn(Py-tzNHC)2m] (n > m), nor mono-carbene-Pd species, or negatively charged Pd containing ions (ESI−) could be found in the spectra.
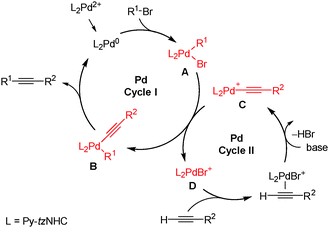 | ||
| Scheme 3 Proposed mechanism and reactive intermediates drawn in red colour as identified by ESI-HRMS. | ||
The proposed plausible mechanism is shown in Scheme 3 and contains two connected Pd-cycles (I and II). Much research work has been devoted to address the question whether mono-ligated Pd0(NHC) or bis-ligated Pd0(NHC)2 is involved in the catalytic cycle.31–33 In our case, the fact that no mono-carbene Pd(Py-tzNHC) species could be found in the ESI-HRMS spectra suggest Pd0(Py-tzNHC)2 to be the catalytically active species. The latter undergoes oxidative addition with aryl bromide to form intermediate Avia an associative mechanism without dissociation of the Py-tzNHC ligand.
The Pd0(Py-tzNHC)2 species may be generated by reductive elimination (alkyne homocoupling) from [PdII(Py-tzNHC)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2)2].34 The species [PdII(Py-tzNHC)2(C
CR2)2].34 The species [PdII(Py-tzNHC)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2)2] was identified by ESI-HRMS as the [M + H]+ ion (m/z 809.2318, calcd for C46H39N8Pd+ 809.2327, ESI‡).
CR2)2] was identified by ESI-HRMS as the [M + H]+ ion (m/z 809.2318, calcd for C46H39N8Pd+ 809.2327, ESI‡).
The acetylene η2-coordination to the bromido-Pd species D and a subsequent base mediated deprotonation produce the alkynylpalladium intermediate C, which then undergoes transmetalation with A to form intermediate B. This was confirmed by an independent ESI-HRMS experiment, where premixing either 1(2BF4) or D, acetylene 3a and Cs2CO3 in DMF at 100 °C resulted in the accumulation of C. Intermediate C completely disappeared from the spectra after the addition of an excess of aryl bromide 2a with the concomitant product 4a formation (ESI‡).
Cation D can be formed independently by reacting 1(2BF4) with KBr. Although we were unable to support the structure of D by single crystal X-ray analysis, this was possible for the closely related cation 5, formed by treating 1(2BF4) with potassium acetate (Fig. 3) (ESI‡).
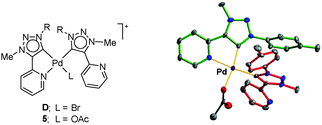 | ||
| Fig. 3 Structures of D and 5 (R = 4-Me-C6H4−), and Ortep drawing of 5 (ESI‡). | ||
In conclusion, a novel type of water soluble and thermally stable Pd-NHC complex 1(2BF4) based on a bidentate pyridyl-1,2,3-triazol-5-ylidene ligand that requires only low synthetic investment has been identified as a highly efficient precatalyst for Sonogashira cross-coupling. We know of no such efficient aryl bromide–terminal acetylene cross-coupling that proceeds in air and in pure water, and in the complete absence of amine, copper, phosphine and other additives, as reported herein for complex 1(2BF4). To our knowledge, this is the first report on the Sonogashira catalysis with a cationic Pd-complex. Preliminary mechanistic investigation indicates that bis-carbene palladium reactive species are involved in two connected palladium catalytic cycles.
Financial support from the Ministry of Education, Science and Sport, Republic of Slovenia, the Slovenian Research Agency (Grant P1-0230; Postdoctoral Grant to M.G. (430-168/2013/114), and Grant P1-0175) is acknowledged. This work was partially supported through the infrastructure of the EN-FIST Centre of Excellence, Ljubljana, Slovenia.
Notes and references
- A. J. Arduengo, R. L. Harlow and M. J. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef CAS.
- W. A. Herrmann, M. Elison, J. Fischer, C. Köcher and G. R. J. Artus, Angew. Chem., Int. Ed. Engl., 1995, 34, 2371 CrossRef CAS.
- R. Jackstell, M. G. Andreu, A. Frisch, K. Selvakumar, A. Zapf, H. Klein, A. Spannenberg, D. Röttger, O. Briel, R. Karch and M. Beller, Angew. Chem., Int. Ed., 2002, 41, 986 CrossRef CAS.
- L. R. Titcomb, S. Caddick, F. G. N. Cloke, D. J. Wilson and D. McKerrecher, Chem. Commun., 2001, 1388 RSC.
- M. S. Viciu, R. F. Germaneau, O. Navarro-Fernandez, E. D. Stevens and S. P. Nolan, Organometallics, 2002, 21, 5470 CrossRef CAS.
- V. César, S. Bellemin-Laponnaz and L. H. Gade, Organometallics, 2002, 21, 5204 CrossRef.
- D. R. Jensen, M. J. Schultz, J. A. Mueller and M. S. Sigman, Angew. Chem., Int. Ed., 2003, 42, 3810 CrossRef CAS PubMed.
- P. Mathew, A. Neels and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 13534 CrossRef CAS PubMed.
- (a) T. Karthikeyan and S. Sankararaman, Tetrahedron Lett., 2009, 50, 5834 CrossRef CAS; (b) T. Nakamura, K. Ogata and S. Fukuzawa, Chem. Lett., 2010, 39, 920 CrossRef CAS; (c) S. Hohloch, W. Frey, C.-Y. Suc and B. Sarkar, Dalton Trans., 2013, 42, 11355 RSC; (d) M. Górna, M. S. Szulmanowicz, A. Gniewek, W. Tylus and A. M. Trzeciak, J. Organomet. Chem., 2015, 785, 92 CrossRef.
- Selected reviews on N-heterocyclic carbenes: (a) J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978 CrossRef CAS PubMed; (b) N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS PubMed; (c) F. A. Glorius, Top. Organomet. Chem., 2007, 21, 1 CrossRef CAS; (d) E. A. B. Kantchev, C. J. O’Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed; (e) O. Kühl, Coord. Chem. Rev., 2009, 253, 2481 CrossRef; (f) M. C. Jahnke and F. E. Hahn, Top. Organomet. Chem., 2010, 30, 95 CrossRef CAS; (g) S. Budagumpi, R. A. Haque and A. W. Salman, Coord. Chem. Rev., 2012, 256, 1787 CrossRef CAS; (h) M. Fèvre, J. Pinaud, Y. Gnanou, J. Vignolle and D. Taton, Chem. Soc. Rev., 2013, 42, 2142 RSC; (i) N-Heterocyclic Carbenes. Effective Tools for Organometallic Synthesis, ed. S. P. Nolan, Wiley-VCH, Weinheim, 2014 Search PubMed; (j) T. A. Schaub and M. Kivala, in Metal-Catalyzed Cross-Coupling Reactions and More, ed. A. de Meijere, S. Bräse and M. Oestreich, Wiley-VCH, Weinheim, 2014, pp. 665–762 Search PubMed; (k) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed.
- (a) A. A. D. Tulloch, A. A. Danopoulos, R. P. Tooze, S. M. Cafferkey, S. Kleinhenz and M. B. Hursthouse, Chem. Commun., 2000, 1247–1248 RSC; (b) D. S. McGuinness and K. J. Cavell, Organometallics, 2000, 19, 741 CrossRef CAS.
- A. A. D. Tulloch, A. A. Danopoulos, G. J. Tizzard, S. J. Coles, M. B. Hursthouse, R. S. Hay-Motherwell and W. B. Motherwell, Chem. Commun., 2001, 1270 RSC.
- V. Khlebnikov, A. Meduri, H. Mueller-Bunz, T. Montini, P. Fornasiero, E. Zangrando, B. Milani and M. Albrecht, Organometallics, 2012, 31, 976 CrossRef CAS.
- C. J. O’Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. Hopkinson and M. G. Organ, Chem. – Eur. J., 2006, 12, 4743 CrossRef PubMed.
- Selected reviews on tzNHCs: (a) O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 CrossRef CAS PubMed; (b) J. D. Crowley, A. Lee and K. J. Kilpin, Aust. J. Chem., 2011, 64, 1118 CrossRef CAS; (c) D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723 RSC; (d) R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755 CrossRef CAS; (e) B. Schulze and U. S. Schubert, Chem. Soc. Rev., 2014, 43, 2522 RSC.
- (a) W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290 CrossRef CAS; (b) M. Melaimi, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 8810 CrossRef CAS PubMed; (c) X.-F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986 RSC; (d) S. Inomata, H. Hiroki, T. Terashima, K. Ogata and S. Fukuzawa, Tetrahedron, 2011, 67, 7263 CrossRef CAS; (e) K. F. Donnelly, A. Petronilho and M. Albrecht, Chem. Commun., 2013, 49, 1145 RSC.
- D. Canseco-Gonzalez, A. Gniewek, M. Szulmanowicz, H. Müller-Bunz, A. M. Trzeciak and M. Albrecht, Chem. – Eur. J., 2012, 18, 6055 CrossRef CAS PubMed.
- J. Huang, J.-T. Hong and S. H. Hong, Eur. J. Org. Chem., 2012, 6630 CAS.
- (a) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (b) K. Sonogashira, in Metal Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1998, pp. 203–229 Search PubMed; (c) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed; (d) M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33 CrossRef CAS; (e) M. Bakherad, Appl. Organomet. Chem., 2013, 27, 125 CrossRef CAS; (f) A. M. Thomas, A. Sujatha and G. Anilkumar, RSC Adv., 2014, 4, 21688 RSC; (g) R. A. D. Arancon, C. S. K. Lin, C. Vargas and R. Luque, Org. Biomol. Chem., 2014, 12, 10 RSC.
- D. Wang and S. Gao, Org. Chem. Front., 2014, 1, 556 RSC.
- R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed.
- (a) A. O. King and N. Yasuda, Top. Organomet. Chem., 2004, 6, 205 CAS; (b) C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef CAS.
- (a) H. D. Velazquez and F. Verpoort, Chem. Soc. Rev., 2012, 41, 7032 RSC; (b) Metal-Catalyzed Reactions in Water, ed. P. Dixneuf and V. Cadierno, Wiley-VCH, Weinheim, 2013 Search PubMed; (c) E. Levin, E. Ivry, C. E. Diesendruck and N. G. Lemcoff, Chem. Rev., 2015, 115, 4607 CrossRef CAS PubMed.
- (a) L. Yang, P. Guan, P. He, Q. Chen, C. Cao, Y. Peng, Z. Shi, G. Pang and Y. Shi, Dalton Trans., 2012, 41, 5020 RSC; (b) A. Kumar and P. Ghosh, Eur. J. Inorg. Chem., 2012, 3955 CrossRef CAS and references therein; (c) C. W. D. Gallop, M.-T. Chen and O. Navarro, Org. Lett., 2014, 16, 3724 CrossRef CAS PubMed.
- A. Bolje and J. Košmrlj, Org. Lett., 2013, 15, 5084 CrossRef CAS PubMed.
- E. C. Keske, O. V. Zenkina, R. Wang and C. M. Crudden, Organometallics, 2012, 31, 6215 CrossRef CAS.
- F. F. Wagner and D. L. Comins, J. Org. Chem., 2006, 71, 8673 CrossRef CAS PubMed.
- R. H. Crabtree, Chem. Rev., 2012, 112, 1536 CrossRef CAS PubMed.
- M. S. Szulmanowicz, A. Gniewek, W. Gil and A. M. Trzeciak, ChemCatChem, 2013, 5, 1152 CrossRef CAS.
- (a) D. Schröder, Acc. Chem. Res., 2012, 45, 1521 CrossRef PubMed; (b) K. L. Vikse, Z. Ahmadi and J. S. McIndoe, Coord. Chem. Rev., 2014, 279, 96 CrossRef CAS.
- J. Pytkowicz, S. Roland, P. Mangeney, G. Meyer and A. Jutand, J. Organomet. Chem., 2003, 678, 166 CrossRef CAS.
- A. K. K. Lewis, S. Caddick, F. G. N. Cloke, N. C. Billingham, P. B. Hitchcock and J. Leonard, J. Am. Chem. Soc., 2003, 125, 10066 CrossRef PubMed.
- (a) U. Christmann and R. Vilar, Angew. Chem., Int. Ed., 2005, 44, 366 CrossRef CAS PubMed; (b) A. Jutand, Chem. Rev., 2008, 108, 2300 CrossRef CAS PubMed; (c) A. Jutand, J. Pytkowicz, S. Roland and P. Mangeney, Pure Appl. Chem., 2010, 82, 1393 CrossRef CAS.
- G. P. McGlacken and I. J. S. Fairlamb, Eur. J. Org. Chem., 2009, 4011 CrossRef CAS.
Footnotes |
| † Dedicated to Dr Maja Osmak on the occasion of her 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, spectra, crystallographic details, and CIF files. CCDC 1405057 and 1430207. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc08717a |
| This journal is © The Royal Society of Chemistry 2016 |