Open Access Article
Open Access ArticleA new family of wurtzite-phase Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) nanocrystals for solar energy conversion applications†
Anima
Ghosh
ab,
Soubantika
Palchoudhury
*a,
Rajalingam
Thangavel
b,
Ziyou
Zhou
a,
Nariman
Naghibolashrafi
a,
Karthik
Ramasamy
*c and
Arunava
Gupta
*a
aCenter for Materials for Information Technology, The University of Alabama, Tuscaloosa, AL, USA. E-mail: agupta@mint.ua.edu; soubantika@gmail.com; Fax: +1-2053482346; Tel: +1-2053483822
bDepartment of Applied Physics, Indian School of Mines, Dhanbad, Jharkhand, India. E-mail: thangavel.r.ap@ismdhanbad.ac.in
cCenter for Integrated Nanotechnologies, Los Alamos National Laboratory, Albuquerque, NM, USA. E-mail: kramasamy@lanl.gov
First published on 2nd October 2015
Abstract
A new family of quaternary semiconductors Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) has been synthesized in the form of wurtzite phase nanocrystals for the first time. The nanocrystals can be converted to the stannite phase via thermal annealing under a N2 atmosphere. A direct band gap in the visible wavelength region combined with a high absorption cross-section makes these materials promising for solar energy conversion applications.
The relentless demand in energy generation through non-fossil fuels inspires the scientific community to develop stable and better performing materials that are composed of sustainable, non-toxic and cost-effective elements.1,2 In this regard, direct band gap I–III–VI2 based ternary semiconductors are a viable alternative to widely used silicon for photovoltaics since they absorb solar radiation more effectively. Energy conversion efficiencies of nearly 20% have been achieved from I–III–VI2 based thin film solar cells.3,4 Despite I–III–VI2 based materials being more cost-effective and showcasing tremendous potential in efficiency improvement, the cost of energy generation is yet to meet grid parity. This has in large part been attributed to the scarcity of indium. Consequently, significant effort has been devoted to the identification of affordable and sustainable alternatives.5–9 In recent years Cu2ZnSnS4 (CZTS), derived by substituting In with abundant Zn and Sn, has been explored as an alternative with significant progress being already realized.10–13 Nevertheless, the energy conversion efficiency of solar cells using CZTS still lags behind that for I–III–VI2 and has not seen an improvement of over 12% in recent years.14 At this juncture, the quest for affordable and sustainable energy generation materials still remains.9,15–17 A possible approach to addressing this issue without sacrificing energy conversion efficiency would be to partially substitute In with Zn.18 Aiming towards this objective, we have for the first time developed a new family of quaternary semiconductors Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) in the form of nanocrystals (NCs). To the best of our knowledge, homogeneous compositions of Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) have not been synthesized previously in any form. Thus far, the closest compositions reported in the literature are for Cu–In–Zn–S wurtzite alloys that are created by alloying different proportions of wurtzite phase CuInS2 and ZnS.19–23 This method of alloying shifts the band gap of the material beyond the useful solar absorption region and thereby diminishes their suitability for solar cells.24 Moreover, the Ga and Al analogues of these alloys have not been reported.
Herein we report the synthesis of Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) semiconductors in the form of wurtzite phase NCs along with detailed electronic structure calculations. The band gap of these newly developed materials is in the visible range, between 1.20 and 1.72 eV, meeting the primary requisite for solar cells. Band structure calculations predict direct band gap characteristics for these quaternary semiconductors with high absorption co-efficients and band gaps closely matching with the experimental values. In addition, we show that the NCs can be readily transformed by annealing from the disordered wurtzite phase to the ordered stannite phase without significantly altering their morphologies and optical properties.
The quaternary composition chalcogenide NCs were synthesized using the colloidal hot-injection method.7 For the synthesis of Cu2ZnInS4−x (CZIS1) NCs, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of acetylacetonate complexes of copper(II), zinc(II) and indium(III) were first heated to 150 °C in oleylamine (OLA) in an inert atmosphere. This was followed by rapid injection of a mixture of n-dodecanethiol (n-DDT, 1 mL) and tert-dodecanethiol (t-DDT, 1 mL) and consequent heating of the solution to 250 °C and maintaining at this temperature for 1 h. The mixture was then cooled and cleaned via two washes in hexane/ethanol to obtain the final NC product. The resulting NCs were readily dispersible in nonpolar solvents like hexane. A similar procedure was used for obtaining the CuZn2InS4 (CZIS2) NCs, but by changing the metal precursor mixture composition to 1
1 ratio of acetylacetonate complexes of copper(II), zinc(II) and indium(III) were first heated to 150 °C in oleylamine (OLA) in an inert atmosphere. This was followed by rapid injection of a mixture of n-dodecanethiol (n-DDT, 1 mL) and tert-dodecanethiol (t-DDT, 1 mL) and consequent heating of the solution to 250 °C and maintaining at this temperature for 1 h. The mixture was then cooled and cleaned via two washes in hexane/ethanol to obtain the final NC product. The resulting NCs were readily dispersible in nonpolar solvents like hexane. A similar procedure was used for obtaining the CuZn2InS4 (CZIS2) NCs, but by changing the metal precursor mixture composition to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Cu
1 (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In). The processes for CZIS1 and CZIS2 NCs were extended for synthesizing the analogous Ga and Al compounds using the respective acetylacetonate precursors. The details of the synthesis methods are provided in the ESI.†
In). The processes for CZIS1 and CZIS2 NCs were extended for synthesizing the analogous Ga and Al compounds using the respective acetylacetonate precursors. The details of the synthesis methods are provided in the ESI.†
The one-step approach described above yields uniform Cu2ZnInS4−x (CZIS1) nanorods of size ∼55 (l) × 10 (w) nm (Fig. 1a). A similar nanorod morphology has been reported in the literature for a number of other wurtzite phase ternary and quaternary chalcogenide NCs.9,10,23 The synthesized CZIS1 NCs are highly crystalline (Fig. 1b). Based on the matching of the inter-fringe distances (0.34 ± 0.01 nm) with the (100) plane of wurtzite, the nanorods likely belong to the wurtzite phase (Fig. 1b). To further investigate the compositional homogeneity of the rod-shaped NCs, energy-dispersive X-ray spectroscopy (EDX) measurements have been performed on different regions within the individual NCs using transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The NCs show a homogeneous composition of Cu2ZnInS4−x (x = 0.5 ± 0.3), closely matching with the stoichiometric ratios (Fig. S1, ESI†). The anion non-stoichiometry seen in these NCs has also been observed for other wurtzite phase chalcogenides, likely to maintain charge neutrality in the compound.25 Unlike CZIS1, the morphology of CuZn2InS4 (CZIS2) NCs is quite distinct, being in the form of ∼2 μm long and ∼27 nm wide nanoworms with slightly curved regions (Fig. 1c). Clear and uniform lattice fringes are also observed in all portions of the worm-like CZIS2 NCs via HRTEM indicating a homogeneous composition and high crystallinity (Fig. 1d, Fig. S2 and S3e, f, ESI†). The inter-fringe distance of 0.33 ± 0.02 nm corresponds to the (100) planes of wurtzite. The average chemical composition of the NCs, as determined from the EDX is CuZn2InS4±0.1, closely related to the stoichiometric amounts and as expected based on charge neutrality. In the syntheses of CZIS NCs of both compositions, a carefully measured 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of n-DDT and t-DDT is essential for stoichiometric and morphological control (Fig. S4, ESI†). The ligand mixture (n-DDT and t-DDT) serves as the sulfur source and is known to passivate the surfaces to preferentially form wurtzite-phase NC.26,27
1 volume ratio of n-DDT and t-DDT is essential for stoichiometric and morphological control (Fig. S4, ESI†). The ligand mixture (n-DDT and t-DDT) serves as the sulfur source and is known to passivate the surfaces to preferentially form wurtzite-phase NC.26,27
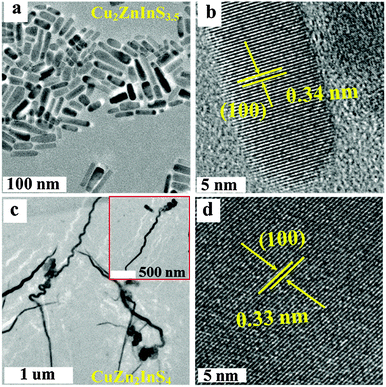 | ||
| Fig. 1 TEM images of CZIS NCs of different compositions. (a) Cu2ZnInS4−x (x = 0.5 ± 0.3) nanorods, (b) high resolution TEM (HRTEM) of (a), (c) CuZn2InS4±0.1 nanoworms, and (d) HRTEM of (c). | ||
This robust synthetic technique can be generalized to CZAlS and CZGS, forming a new class of I–II–III–VI chalcogenide NCs (Fig. S5, ESI†). The respective acetylacetonate precursors are particularly chosen for their low decomposition temperature and specific reactivity.9,28,29 Uniform and nearly spherical NCs of high crystallinity and size ∼27 nm can be formed for both Cu2ZnAlS4−x (x = 0.5 ± 0.3) and CuZn2AlS4±0.1 compositions (Fig. S3a and b, ESI†). In contrast, the Cu2ZnGaS4−x (x = 0.5 ± 0.3) and CuZn2GaS4±0.1 NCs of size ∼45 nm show a tadpole-like morphology (Fig. S3c and d, ESI†). Interestingly, a somewhat higher reaction temperature (300 °C) is required for these NCs as compared to the CZIS. A possible explanation can be in terms of the difference in the ionic radius since the size of the cation is known to play a key role in the phase and size evolution of NCs.30 CZAlS and CZGS NCs also exhibit the wurtzite phase, based on the inter-fringe distances.
Phase-pure quaternary Cu2ZnAS4−x and CuZn2AS4 NCs have thus far been synthetically challenging to obtain. The primary impediment is the formation of stable binary phases, which should be prevented. Fig. 2a shows powder X-ray diffraction (XRD) measurements confirming the pure wurtzite phases of Cu2ZnAS4−x NCs. The blue lines indicate the simulated patterns (CaRIne crystallography), considering CZAS as cation-disordered derivatives of the wurtzite ZnS structure, since no standard XRD pattern exists in the database for this family of NCs. XRDa3.1 software is used to match the experimental XRD peaks. These experimental peaks can be indexed to the (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes of pure wurtzite phase [space group P63mc (No. 186)], in close match with the derived simulated pattern. Based on a comparison of XRD peaks, the NCs are free of any binary phase impurities (Fig. S6, ESI†). The lattice parameter ratio c/a, as determined from the diffraction peaks is ∼1.6, similar to literature reported values for the wurtzite phase of CZTS (Table S1, ESI†). In addition, the pure wurtzite phase is also obtained for the CuZn2AS4 NC compositions (Fig. S7a, ESI†), leading to a new class of I–II–III–VI wurtzite phase NCs. In general, wurtzite is a cation-disordered metastable phase formed at lower reaction temperatures, while the structurally related ordered stannite/kesterite phases are stable forms at higher temperatures.24,31,32 To exploit the full potential of our synthetic method, a facile phase transformation approach is investigated for CZAS NCs. The Cu2ZnAS4−x NCs can be transformed to the pure stannite phase via annealing at 400 °C for 2–2.5 h in a N2 atmosphere, as indicated from the XRD analyses (Fig. 2b).33 A higher temperature annealing (500 °C) is required for the CuZn2AS4 NCs (Fig. S7b, ESI†). In addition, Rietveld refinement performed on the experimental XRD pattern shows a good fit for the wurtzite and stannite phases (Fig. S8, ESI† CuZn2AS4±0.1).
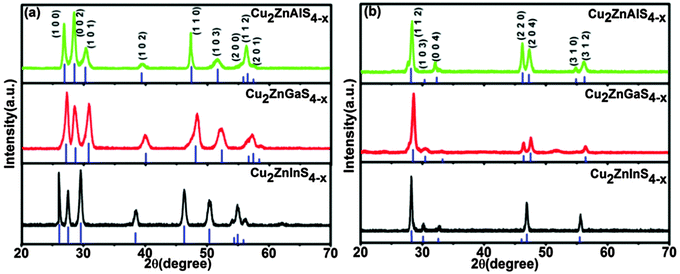 | ||
| Fig. 2 XRD plots showing the crystal phase of Cu2ZnAS4−x (x = 0.5 ± 0.3) NCs. (a) Pure wurtzite phase and (b) pure stannite phase after annealing under N2 atmosphere. | ||
X-ray photoelectron spectroscopy (XPS) provides a suitable complement to EDX for chemical composition analysis as it can determine the oxidation states of the constituent elements on the surface of NCs. Fig. S9 (ESI†) shows the representative high resolution XPS pattern for Cu2ZnInS4−x (x = 0.5 ± 0.3) NCs. The Cu2p core-spectrum shows two major peaks at 931.4 eV (2p3/2) and 951.2 eV (2p1/2), with a peak splitting of 20.0 eV, indicative of monovalent Cu.10 The ligand n-DDT is likely responsible for the reduction of Cu(acac)2 to Cu(I).34 Zn2p peaks appear at binding energies 1021.1 eV (2p3/2) and 1044.3 eV (2p1/2), characteristic of Zn(II) since the peak separation is 22.9 eV (Fig. S9b, ESI†).10,35 Fig. S9c (ESI†) shows the In3d spectrum with contributions from 3d5/2 and 3d3/2 at 444.6 eV and 452.2 eV indicating a spin–orbit splitting of 7.6 eV, characteristic of In(III).36 The sulfur spectrum with peaks at binding energies 162.2 eV (2p3/2) and 163.2 eV (2p1/2) and a doublet separation of 1.1 eV can be attributed to the presence of S2− (Fig. S9d, ESI†).37 The XPS spectrum of CuZn2AS4 NCs also shows similar oxidation states of the elements.
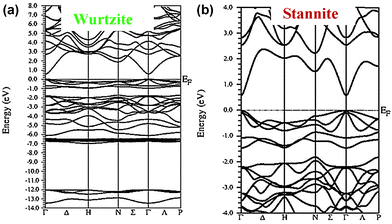
To investigate the optical properties of the new class of phase-pure wurtzite NCs, ultraviolet-visible spectroscopy (UV-vis) measurements are performed on well-dispersed NC solutions in hexane (Fig. 3a). The direct optical band gaps (Eg,opt) are determined from the absorbance spectra onset through the extrapolation of the linear portion of the (Ahν)2versus hν (A = absorbance, h = Planck's constant, and ν = frequency) plot in the band edge region (Fig. 3b). The band gaps are determined to be 1.78 ± 0.05, 1.64 ± 0.04, and 1.42 ± 0.03 eV for CuZn2AlS4±0.1, CuZn2GaS4±0.1, and CuZn2InS4±0.1, respectively. There is a decrease in the band gap from Al to In, suggesting a likely effect of the increasing ionic radius. Similar band gaps in the visible wavelength range are observed for the Cu2ZnAS4−x (x = 0.5 ± 0.3) NCs (Fig. S10 and S11, ESI†). For assessing the applicability of these newly developed materials as absorber layers in solar cells, it is important to gain a better understanding of their optical properties. For this purpose we have carried out density functional theory (DFT) calculations using the full-potential linearized augmented plane wave plus local orbital (FP-LAPW+lo) method, as implemented in the WIEN2K code (see the ESI† for details).38 Our calculations predict a direct band gap transition in both the wurtzite and stannite phases at the Γ point for all CuZn2AS4 compositions (Fig. 4). The band gap values for the wurtzite phase, estimated from the first principle calculations scissor operator, are 1.71 eV for CuZn2AlS4, 1.34 eV for CuZn2GaS4, and 1.26 eV for CuZn2InS4 (Fig. 3c). These values, along with the decreasing trend from Al to In, are in good agreement with the experimental data. Moreover, the absorption coefficient in the visible wavelength region, which is an important parameter for thin film solar cells, is calculated to be over 104 cm−1 for these compounds, similar to that for CIGS and CZTS.7,9,10
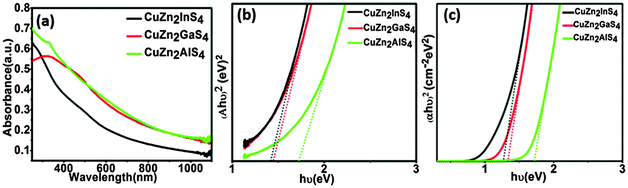 | ||
| Fig. 3 Band gap measurement of CuZn2AS4±0.1 NCs. (a) UV-vis absorption spectra, (b) experimental Tauc plots, and (c) theoretically calculated Tauc plots. | ||
In summary, we have synthesized a new family of quaternary semiconductors Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) in the form of nanocrystals. The NCs are synthesized using the colloidal hot-injection method wherein a mixture of thiols is injected into a vessel containing a solution of the metal precursors at an elevated temperature. We have obtained wurtzite phase NCs with distinct morphologies from nanorods, and nanoworms, to nanotadpoles. These exhibit a homogeneous composition, based on EDX, TEM, and XPS. In addition, phase transformation of the NCs from wurtzite to stannite can be induced via annealing under N2. Importantly, these new compositions are direct band gap materials having band gaps between 1.20 eV and 1.72 eV with a high absorption cross-section, as confirmed from experimental absorption measurements and theoretical calculations. Our initial investigations indicate that these materials possess the requisite optical characteristics to be used as cost-effective and nontoxic absorber layers in solar cell applications. Nevertheless, the full potential can only be confirmed after investigating their charge transport characteristics in solar cell devices, which is being pursued actively in our group and the results will be presented elsewhere.
This work was supported by the US DOE, Office of Basic Energy Sciences, Div. Material Sciences and Eng. Award DE-FG02-08ER46537. A. Ghosh was supported by a Bhaskara Advanced Solar Energy Fellowship of Indo-US Sci & Tech Forum. The authors thank UA-CAF for TEM, SEM, and XPS and UA-Geology Dept. for XRD. The authors thank Rob Holler for XPS measurements. The authors acknowledge UA-MINT and ISM, Dhanbad.
Notes and references
- D. B. Mitzi, O. Gunawan, T. K. Todorov, K. Wang and S. Guha, Sol. Energy Mater. Sol. Cells, 2011, 95, 1421 CrossRef CAS.
- S. E. Habas, H. A. S. Platt, M. F. A. M. van Hest and D. S. Ginley, Chem. Rev., 2010, 110, 6571 CrossRef CAS PubMed.
- T. Saga, NPG Asia Mater., 2010, 2, 96 CrossRef.
- L. Li, A. Pandey, D. J. Werder, B. P. Khanal, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2011, 133, 1176 CrossRef CAS PubMed.
- K. Ramasamy, H. Sims, W. H. Butler and A. Gupta, J. Am. Chem. Soc., 2014, 136, 1587 CrossRef CAS PubMed.
- K. Ramasamy, M. A. Malik, N. Revaprasadu and P. O'Brien, Chem. Mater., 2013, 25, 3551 CrossRef CAS.
- X. Zhang, N. Bao, K. Ramasamy, Y. H. A. Wang, Y. Wang, B. Lin and A. Gupta, Chem. Commun., 2012, 48, 4956 RSC.
- K. Ramasamy, X. Zhang, R. D. Bennett and A. Gupta, RSC Adv., 2013, 3, 1186 RSC.
- Y.-H. A. Wang, X. Zhang, N. Bao, B. Lin and A. Gupta, J. Am. Chem. Soc., 2011, 133, 11072 CrossRef CAS PubMed.
- A. Singh, H. Geaney, F. Laffir and K. M. Ryan, J. Am. Chem. Soc., 2012, 134, 2910 CrossRef CAS PubMed.
- K. Ramasamy, M. A. Malik and P. O'Brien, Chem. Commun., 2012, 48, 5703 RSC.
- K. Ramasamy, M. A. Malik and P. O'Brien, Chem. Sci., 2011, 2, 1170 RSC.
- J.-J. Wang, P. Liu and K. M. Ryan, Chem. Commun., 2015, 51, 13810 RSC.
- J. Kim, H. Hiroi, T. K. Todorov, O. Gunawan, M. Kuwahara, T. Gokmen, D. Nair, M. Hopstaken, B. Shin, Y. S. Lee, W. Wang, H. Sugimoto and D. B. Mitzi, Adv. Mater., 2014, 26, 7427 CrossRef CAS PubMed.
- N. Guijarro, E. Guillen, T. Lana-Villarreal and R. Gomez, Phys. Chem. Chem. Phys., 2014, 16, 9115 RSC.
- F.-J. Fan, L. Wu, M. Gong, S. Y. Chen, G. Y. Liu, H.-B. Yao, H.-W. Liang, Y.-X. Wang and S.-H. Yu, Sci. Rep., 2012, 2, 952 Search PubMed.
- J.-J. Wang, J.-S. Hu, Y.-G. Guo and L.-J. Wan, NPG Asia Mater., 2012, 4, e2 CrossRef.
- S. Chen, X. G. Gong, A. Walsh and S.-H. Wei, Phys. Rev. B, 2009, 79, 165211 CrossRef.
- C. Ye, M. D. Regulacio, S. H. Lim, Q.-H. Xu and M.-Y. Han, Chem. – Eur. J., 2012, 18, 11258 CrossRef CAS PubMed.
- C. Ye, M. D. Regulacio, S. H. Lim, S. Li, Q.-H. Xu and M.-Y. Han, Chem. – Eur. J., 2015, 21, 9514 CrossRef CAS PubMed.
- S. Cao, C. Li, L. Wang, M. Shang, G. Wei, J. Zheng and W. Yang, Sci. Rep., 2014, 4, 7510 CrossRef PubMed.
- L. De Trizio, M. Prato, A. Genovese, A. Casu, M. Povia, R. Simonutti, M. J. P. Alcocer, C. D'Andrea, F. Tassone and L. Manna, Chem. Mater., 2012, 24, 2400 CrossRef CAS.
- A. Singh, C. Coughlan, D. J. Milliron and K. M. Ryan, Chem. Mater., 2015, 27, 1517 CrossRef CAS.
- R. Mainz, A. Singh, S. Levcenko, M. Klaus, C. Genzel, K. M. Ryan and T. Unold, Nat. Commun., 2014, 5, 3133 CAS.
- X. Zhang, N. Bao, B. Lin and A. Gupta, Nanotechnology, 2013, 24, 105706 CrossRef PubMed.
- U. Ghorpade, M. Suryawanshi, S. W. Shin, K. Gurav, P. Patil, S. Pawar, C. W. Hong, J. H. Kim and S. Kolekar, Chem. Commun., 2014, 50, 11258 RSC.
- X. Lu, Z. Zhuang, Q. Peng and Y. Li, Chem. Commun., 2011, 47, 3141 RSC.
- M. A. Franzman, V. Perez and R. L. Brutchey, J. Phys. Chem. C, 2009, 113, 630 CAS.
- Y. Zou, X. Su and J. Jiang, J. Am. Chem. Soc., 2013, 135, 18377 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- X. Shen, E. A. Hernandez-Pagan, W. Zhou, Y. S. Puzyrev, J.-C. Idrobo, J. E. Macdonald, S. J. Pennycook and S. T. Pantelides, Nat. Commun., 2014, 5, 5431 CrossRef CAS PubMed.
- S. Chen, A. Walsh, Y. Luo, J.-H. Yang, X. G. Gong and S.-H. Wei, Phys. Rev. B, 2010, 82, 195203 CrossRef.
- A. Shavel, J. Arbiol and A. Cabot, J. Am. Chem. Soc., 2010, 132, 4514 CrossRef CAS PubMed.
- J.-J. Wang, D.-J. Xue, Y.-G. Guo, J.-S. Hu and L.-J. Wan, J. Am. Chem. Soc., 2011, 133, 18558 CrossRef CAS PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray photoelectron spectroscopy, Perkin Elmer, Eden Prairie, MN, 1992 Search PubMed.
- H. Virieux, M. Le Troedec, A. Cros-Gagneux, W.-S. Ojo, F. Delpech, C. Nayral, H. Martinez and B. Chaudret, J. Am. Chem. Soc., 2012, 134, 19701 CrossRef CAS PubMed.
- V. Lesnyak, C. George, A. Genovese, M. Prato, A. Casu, S. Ayyappan, A. Scarpellini and L. Manna, ACS Nano, 2014, 8, 8407 CrossRef CAS PubMed.
- P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka and J. Luitz, WIEN2k, An Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties, Karlheinz Schwarz, Techn. University at Wien, Austria, 2001 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and theoretical data; SEM, STEM, & EDX: Cu2ZnInS4−x NCs; TEM & HRTEM of CZAS NCs; XRD: binary phases; summary table for structural and optical properties; XRD: CuZn2AS4 NCs; Rietveld: Cu2ZnGaS4−x NCs; XPS: Cu2ZnInS4−x NCs; and band gap plots: Cu2ZnAS4−x. See DOI: 10.1039/c5cc07743e |
| This journal is © The Royal Society of Chemistry 2016 |