A highly enantioselective thiolation of sulfonyl indoles to access 3-sec-sulfur-substituted indoles in water†
Ping
Chen
ab,
Sheng-mei
Lu
a,
Wengang
Guo
ab,
Yan
Liu
*a and
Can
Li
*a
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: yanliu503@dicp.ac.cn; canli@dicp.ac.cn
bGraduate University of Chinese Academy of Sciences, Beijing 100049, China
First published on 26th October 2015
Abstract
A highly enantioselective approach for the synthesis of 3-alkyl- indole or indoline derivatives with a functional thiol group is presented. The chemistry is based on the asymmetric 1,4-addition of thiol to vinylogous imine intermediates, which are generated in situ from sulfonylindoles. The broad substrate transformation proceeds with high yields (up to 96%) and enantioselectivity (up to 98% ee) in a water-compatible system.
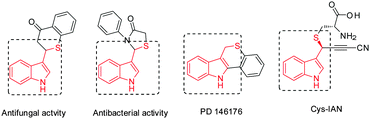
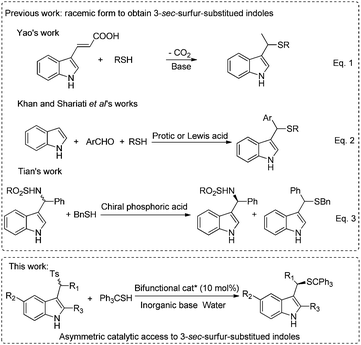
Indoles and their derivatives are ubiquitously found in natural alkaloids, agrochemicals and pharmaceuticals, therefore, considerable efforts have been made to construct or functionalize indole cores.1 Among them, fused heterocyclic substances including both sulfur atom and indole moieties are verified to be biologically active examples including antioxidant, antibacterial, anti-insecticidal and anticancer activity.2 In this context, methodologies for the synthesis of indole thiolethers have been developed using different sulfenylating agents.3 However, as a significant class of biologically active compounds (Fig. 1),4 methods for incorporating sulfur atoms in 3-subsititued indoles have been rarely reported and all of them are racemic.5 Yao and coworkers developed tandem 1,4-addition-decarboxylation between thiols and 3-indoleacrylic acids at high temperature (Scheme 1, eqn (1)).5a Later, Khan and Shariati reported three-component reactions of indoles, aromatic aldehydes and thiols in the presence of a protic or Lewis acid (Scheme 1, eqn (2)).5b,c In Tian's work, N-benzylic sulfonamides underwent kinetic resolution with benzyl thiol, yielding a racemic thioether (Scheme 1, eqn (3)).5d Considering the biological importance and synthetic challenges of enantioenriched 3-sec-sulfur-substituted indoles, developing efficient methods for accessing these compounds is in high demand.
In 2006, Petrini and co-workers reported a privileged 3-substituted sulfonylindole structural skeleton, which can generate vinylogous imine electrophiles under basic conditions.6 Since the sulfa-Michael addition (SMA) reaction is one of the most powerful strategies for asymmetric new C–S bond formation,7 it is supposed that chiral sulfur-containing 3-alkylindoles can be synthesized by the asymmetric 1,4-addition of thiol to vinylogous imines. In fact, some studies on asymmetric additions of different carbon nucleophiles to vinylogous imine intermediates using metal-catalysts, chiral phase-transfer catalysts, and organocatalysts have been well documented.8 Despite these important advances, the asymmetric catalytic addition of thiol nucleophiles to vinylogous imines generated in situ from sulfonylindoles, to the best of our knowledge, has not been reported. The major difficulty may be the severe racemic background reaction caused by the strong nucleophilicity of thiols.
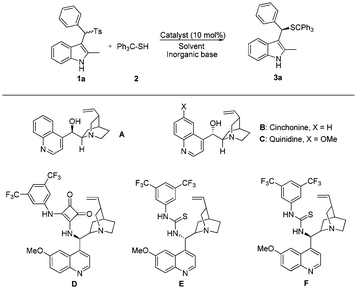
Recently, we developed the organic base catalyzed asymmetric catalytic thiol-addition to in situ generated ortho-quinone methides in the presence of water.9 In this reaction, the racemic background reaction was subdued in the presence of water. In 2010, Johnston et al. reported that enantioselectivity can be improved by the addition of water in the reaction of nitroalkanes with sulfonylindoles.8c To further demonstrate the advantage of the water–oil biphasic system, we report herein the first conjugate addition of tritylthiol to in situ generated vinylogous imines catalyzed by a chiral organic base with excellent enantioselectivities (87–98% ee.) using water as the solvent (Scheme 1). Moreover, experimental results indicate that water is crucial for achieving the high reactivity and stereoselectivity.
At the outset of this work, sulfonylindoles 1a and tritylthiol 2 were chosen as the model substrates. When the reactions were carried out in an organic solvent like those reported in the literature, the reaction was rather slow with only 31% conversion and moderate enantioselectivity of 48% in the presence of cinchonine as the chiral catalyst and Na2CO3 as the base (Table 1, entry 1). By using Et3N as the base in an organic solvent, the homogeneous system was pushed to higher reactivity but low enantioselectivity (Table 1, entry 2). Gratefully, when we chose mixture of H2O/CH2Cl2 (solvent v/v = 1/1) as the solvent, the reaction was dramatically accelerated to complete conversion in 12 hours with a moderate ee value (Table 1, entry 3). Encouraged by these results, we turned our attention to catalyst screening in the presence of water.10 Natural Cinchona alkaloids gave poor enantioselectivities albeit with nearly full conversions (Table 1, entries 5 and 6). Then we began to investigate Cinchona alkaloid-derived squaramide and thiourea catalysts,11 considering the strong interactions between the substrates and the catalyst due to hydrogen bonding. Quinidine-derived squaramide D gave disappointing results regarding enantioselectivity compared to simple Cinchona alkaloid cinchonine. Nevertheless, the Cinchona alkaloid-derived thiourea catalysts E and F showed better performance. The quinidine-derived thiourea catalyst could offer higher enantioselectivity (88% ee vs. 85% ee) than the cinchonine-derived thiourea catalyst (Table 1, entries 8 and 9).
| Entry | Solvent | Cat. | Base | Convb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2 (0.2 mmol, 2 equiv.), catalyst (10 mol%) and base (0.2 mmol, 2 equiv.) in 1 mL solvent at room temperature for 12 h, the mixture solvent v/v = 1/1. b Determined by 1H NMR spectroscopy of the crude mixture. c Determined by chiral stationary phase HPLC analysis. d The reaction was performed at 0 °C. e The reaction was performed with 1a (0.2 mmol) and 2 (0.22 mmol 1.1 equiv.) in H2O (4 mL) and CHCl3 (200 μL) for 12 h. | |||||
| 1 | DCM | A | Na2CO3 | 31 | 48 |
| 2 | DCM | A | Et3N | 66 | 6 |
| 3 | H2O/DCM | A | Na2CO3 | >95 | 66 |
| 4 | H2O/DCM | — | Na2CO3 | 49 | — |
| 5 | H2O/DCM | B | Na2CO3 | >95 | 48 |
| 6 | H2O/DCM | C | Na2CO3 | >95 | 29 |
| 7 | H2O/DCM | D | Na2CO3 | >95 | 50 |
| 8 | H2O/DCM | E | Na2CO3 | >95 | 85 |
| 9 | H2O/DCM | F | Na2CO3 | >95 | 88 |
| 10 | H2O/DCM | F | K2CO3 | >95 | 88 |
| 11 | H2O/DCM | F | NaHCO3 | >95 | 87 |
| 12 | H2O/DCM | F | NaOH | >95 | 86 |
| 13 | H2O/DCM | F | KOH | >95 | 84 |
| 14 | H2O/DCM | F | Cs2CO3 | >95 | 82 |
| 15 | H2O/CHCl3 | F | Na2CO3 | >95 | 89 |
| 16d | H2O/CHCl3 | F | Na2CO3 | >95 | 91 |
| 17d | H2Oe | F | Na2CO3 | >95 | 91 |
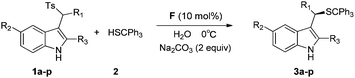
Further screening of inorganic bases suggested that Na2CO3 was the optimal inorganic base in terms of conversions and enantioselectivity. The desired product with a better ee value (89% ee) was obtained when methylene chloride was replaced with chloroform (Table 1, entry 15). Subsequently, the effect of temperature was investigated. When the temperature was lowered to 0 °C, the enantioselectivity could be further improved to 91% ee (Table 1, entry 16). When the amount of substrate 2 was reduced to 1.1 equivalents and with water as the solvent (200 μL CHCl3 added to dissolve the substrates), the conversion and enantioselectivity were maintained (Table 1, entry 17). Using 1a as the substrate, other different thiol nucleophiles were investigated under standard conditions. However, moderate enantioselectivities were obtained albeit with almost quantitative yields (see ESI,† P6).
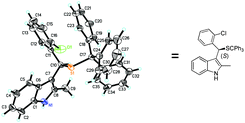
Subsequently, we investigated a series of sulfonylindoles 1 to establish the generality of this asymmetric methodology. As listed in Table 2, by using a catalytic amount of F, the asymmetric thiol addition with most sulfonylindoles afforded the desired products with excellent yields and ee values under optimized conditions. Notably, in the substrates, R1 could be different substituents with phenyl, 1-naphthyl or alkyl groups. A substituent at different positions of the aryl moiety (R1) had a distinct impact on the enantioselectivity (Table 2, entries 2–4). Those substrates with an ortho substituted phenyl ring (R1) gave better enantioselectivities (entry 3 vs. entries 2 and 4). An electron-donating (Me) or electron-withdrawing (F, Br, CF3) substituent on the aryl moiety (R1) fit well to furnish the corresponding products (Table 2, entries 2–11). Fortunately, alkyl-substituted (R1) sulfonylindoles were also demonstrated to be acceptors amenable to the reaction protocol, giving satisfying enantiomeric excesses (96% ee and 98% ee) with acceptable yields (Table 2, entries 12 and 13). Substrate 1n bearing the 2-thienyl group at the R1 position can also achieve good enantioselectivity (87% ee) with an almost quantitative yield (Table 2, entry 14). Variations in the electronic nature of the indole core had little effect on the outcome when sulfonylindole substrates tolerated fluorine or methyl as the substituents at the 5-position of the indole ring (Table 2, entries 15 and 16). When the R3 group was changed from methyl to phenyl, lower reactivity was observed with moderate yield (57% within four days) while the enantioselectivity was maintained (Table 2, entry 17). The absolute configuration of the stereocenter of the Michael addition product 3c was unambiguously assigned to be S by single-crystal X-ray diffraction analysis (see Fig. 2) and those of others were assigned by analogy.
| Entry | R1/R2/R3 | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.2 mmol), tritylthiol (1.1 equiv., 0.22 mmol) and catalyst F (0.02 mmol, 10 mol%) in water 4 mL at 0 °C, 200 μL CHCl3 was added to dissolve the substrates. b Isolated yield based on 1. c Determined by chiral stationary phase HPLC analysis. d Because of the insolubility of substrates, solvent: CHCl3 (2 mL) and H2O (2 mL). | ||||
| 1 | Ph/H/Me | 12 | 3a, 95 | 91 |
| 2 | 4-ClC6H4/H/Me | 12 | 3b, 96 | 91 |
| 3 | 2-ClC6H4/H/Me | 24 | 3c, 91 | 96 |
| 4d | 3-ClC6H4/H/Me | 48 | 3d, 90 | 88 |
| 5 | 4-FC6H4/H/Me | 36 | 3e, 92 | 91 |
| 6 | 4-BrC6H4/H/Me | 12 | 3f, 95 | 91 |
| 7 | 4-CF3C6H4/H/Me | 24 | 3g, 82 | 98 |
| 8 | 4-MeC6H4/H/Me | 12 | 3h, 75 | 91 |
| 9 | 2-MeC6H4/H/Me | 12 | 3i, 90 | 92 |
| 10d | 2-NO2C6H4/H/Me | 36 | 3j, 96 | 98 |
| 11 | 1-Naphthyl/H/Me | 12 | 3k, 96 | 88 |
| 12 | iBu/H/Me | 60 | 3l, 79 | 96 |
| 13 | n Bu/H/Me | 60 | 3m, 75 | 97 |
| 14 | 2-Thienyl/H/Me | 12 | 3n, 99 | 87 |
| 15d | Ph/F/Me | 48 | 3o, 96 | 91 |
| 16 | Ph/Me/Me | 12 | 3p, 95 | 89 |
| 17d | Ph/H/Ph | 96 | 3q, 57 | 91 |
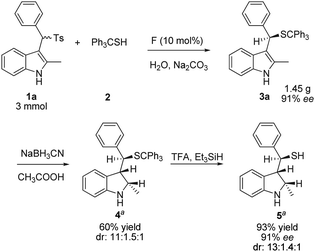
The synthetic utility of this method was demonstrated by the efficient asymmetric synthesis of 3-sec-substituted indoline thiol, starting from the enantioenriched 3a obtained from a gram-scale reaction using the above procedure with F as the catalyst (Scheme 2). The diastereoselective reduction of the indole ring with NaBH3CN under the conditions of acetic acid, followed by the removal of the trityl group, readily delivered chiral mercaptan 5 without the loss of enantiopurity.
In conclusion, we have developed the first thiol-addition to vinylogous imines generated in situ from sulfonylindoles in a water-compatible system. Compared with organic solvents, the aqueous medium showed a remarkable increase in the activity and enantioselectivity for this asymmetric reaction. This eco-friendly organocatalytic protocol provides efficient and convenient access to valuable chiral sulfur-containing 3-sec-substituted indoles in moderate to high yields with excellent enantioselectivity.
This work is financially supported by the National Natural Science Foundation of China (21322202, 21532006).
Notes and references
- For selected reviews on synthesis and functionalization of indoles: (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2005, 106, 2875 CrossRef PubMed; (c) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (d) L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673 CrossRef CAS; (e) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (f) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, 215 CrossRef PubMed; (g) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC.
- (a) G. La Regina, M. C. Edler, A. Brancale, S. Kandil, A. Coluccia, F. Piscitelli, E. Hamel, G. De Martino, R. Matesanz, J. F. Díaz, A. I. Scovassi, E. Prosperi, A. Lavecchia, E. Novellino, M. Artico and R. Silvestri, J. Med. Chem., 2007, 50, 2865 CrossRef CAS PubMed; (b) P. C. Unangst, D. T. Connor, S. R. Stabler, R. J. Weikert, M. E. Carethers, J. A. Kennedy, D. Thueson, J. C. Chestnut, R. L. Adolphson and M. C. Conroy, J. Med. Chem., 1989, 32, 1360 CrossRef CAS PubMed; (c) R. Silvestri, G. De Martino, G. La Regina, M. Artico, S. Massa, L. Vargiu, M. Mura, A. G. Loi, T. Marceddu and P. L. Colla, J. Med. Chem., 2003, 46, 2482 CrossRef CAS PubMed ; For books, see; (d) C. Chatgilialoglu and K. D. Asmus, Sulfur-Centered Reactive Intermediates in Chemistry and Biology, Springer, New York, 1991 Search PubMed; (e) R. J. Sundberg, Indoles, Academic Press, San Diego, 1996 Search PubMed.
- For selected samples, see: (a) J. A. Campbell, C. A. Broka, L. Gong, K. A. M. Walker and J.-H. Wang, Tetrahedron Lett., 2004, 45, 4073 CrossRef CAS; (b) P. Hamel, J. Org. Chem., 2002, 67, 2854 CrossRef CAS PubMed; (c) K. M. Schlosser, A. P. Krasutsky, H. W. Hamilton, J. E. Reed and K. Sexton, Org. Lett., 2004, 6, 819 CrossRef CAS PubMed; (d) P. Sang, Z. Chen, J. Zou and Y. Zhang, Green Chem., 2013, 15, 2096 RSC; (e) Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC; (f) F.-L. Yang and S.-K. Tian, Angew. Chem., Int. Ed., 2013, 52, 4929 CrossRef CAS PubMed; (g) M. Tudge, M. Tamiya, C. Savarin and G. R. Humphrey, Org. Lett., 2006, 8, 565 CrossRef CAS PubMed; (h) M. Matsugi, K. Murata, K. Gotanda, H. Nambu, G. Anilkumar, K. Matsumoto and Y. J. Kita, J. Org. Chem., 2001, 66, 2434 CrossRef CAS PubMed; (i) A. R. Katritzky and K. Akutagawa, Tetrahedron Lett., 1985, 26, 5935 CrossRef CAS; (j) T. Hostier, V. Ferey, G. Ricci, D. G. Pardo and J. Cossy, Chem. Commun., 2015, 51, 13898 RSC.
- (a) A. P. Klein, G. Anarat-Cappillino and E. S. Sattely, Angew. Chem., Int. Ed., 2013, 52, 13625 CrossRef CAS PubMed; (b) D. M. Tobin, F. J. Roca, S. F. Oh, R. McFarland, T. W. Vickery, J. P. Ray, D. C. Ko, Y.-X. Zou, N. D. Bang, T. T. H. Chau, J. C. Vary, T. R. Hawn, S. J. Dunstan, J. J. Farrar, G. E. Thwaites, M.-C. King, C. N. Serhan and L. Ramakrishnan, Cell, 2012, 148, 434 CrossRef CAS PubMed; (c) R. Saundaneanand, Prabhakerwalmik, N. M. Kirankumar and H. Annapurna, Int. J. Pharm. Pharm. Sci., 2014, 6, 141 Search PubMed; (d) A. R. Saundane, M. Yarlakatti, W. Prabhaker and V. Katkar, J. Chem. Sci., 2012, 124, 469 CrossRef CAS; (e) A. P. G. Nikalje, S. I. Shaikh, A. Mulay, F. A. K. Khan, J. N. Sangshetti and S. Shaikh, Arch. Pharm. Chem. Life Sci., 2014, 347, 756 CrossRef CAS PubMed; (f) Y.-L. Song, F. Wua, C.-C. Zhang, G.-C. Liang, G. Zhou and J.-J. Yu, Bioorg. Med. Chem. Lett., 2015, 25, 259 CrossRef CAS PubMed; (g) T. Okubo, R. Yoshikawa, S. Chaki, S. Okuyama and A. Nakazato, Bioorg. Med. Chem., 2004, 12, 3569 CrossRef CAS PubMed; (h) A. R. Saundane and K. N. Mathada, Monatsh. Chem., 2015, 146, 1751 CrossRef CAS; (i) R. S. Vardanyan and V. J. Hruby, Synthesis of essential drugs, Elsevier, Amsterdam, 2006 Search PubMed.
- (a) S.-J. Gao, C. Tseng, B. R. Raju, C.-H. Tsai and C.-F. Yao, Synlett, 2009, 3201 CAS; (b) A. A. Dar, S. Ali and A. T. Khan, Tetrahedron Lett., 2014, 55, 486 CrossRef CAS; (c) A. Khorshidi and S. Shariati, RSC Adv., 2014, 4, 41469 RSC; (d) X.-S. Wu and S.-K. Tian, Chem. Commun., 2012, 48, 898 RSC.
- R. Ballini, A. Palmieri, M. Petrini and E. Torregiani, Org. Lett., 2006, 8, 4093 CrossRef CAS PubMed.
- For reviews, see: (a) D. Enders, K. Luttgen and A. Narine, Synthesis, 2007, 959 CrossRef CAS; (b) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807 CrossRef CAS PubMed ; For selected examples about asymmetric sulfa-Michael additions, see: ; (c) E. Emori, T. Arai, H. Sasai and M. Shibasaki, J. Am. Chem. Soc., 1998, 120, 4043 CrossRef CAS; (d) P. McDaid, Y. Chen and L. Deng, Angew. Chem., Int. Ed., 2002, 41, 338 CrossRef CAS; (e) M. Marigo, T. Schulte, J. Franzén and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 15710 CrossRef CAS PubMed; (f) S. Brandau, E. Maerten and K. A. Jørgensen, J. Am. Chem. Soc., 2006, 128, 14986 CrossRef CAS PubMed; (g) W. Wang, H. Li, J. Wang and L.-S. Zu, J. Am. Chem. Soc., 2006, 128, 10354 CrossRef CAS PubMed; (h) Y. Liu, B.-F. Sun, B.-M. Wang, M. Wakem and L. Deng, J. Am. Chem. Soc., 2009, 131, 418 CrossRef CAS PubMed; (i) K. L. Kimmel, M. T. Robak and J. A. Ellman, J. Am. Chem. Soc., 2009, 131, 8754 CrossRef CAS PubMed; (j) X. Tian, C. Cassani, Y.-K. Liu, A. Moran, A. Urakawa, P. Galzerano, E. Arceo and P. Melchiorre, J. Am. Chem. Soc., 2011, 133, 17934 CrossRef CAS PubMed.
- For selected examples, see: (a) R. R. Shaikh, A. Mazzanti, M. Petrini, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2008, 47, 8707 CrossRef CAS PubMed; (b) Y. Li, F.-Q. Shi, Q.-L. He and S.-L. You, Org. Lett., 2009, 11, 3182 CrossRef CAS PubMed; (c) M. C. Dobish and J. N. Johnston, Org. Lett., 2010, 12, 5744 CrossRef CAS PubMed; (d) L.-H. Jing, J.-T. Wei, L. Zhou, Z.-Y. Huang, Z.-K. Li, D. Wu, H.-F. Xiang and X.-G. Zhou, Chem. – Eur. J., 2010, 16, 10955 CrossRef CAS PubMed; (e) B.-H. Zheng, C.-H. Ding, X.-L. Hou and L.-X. Dai, Org. Lett., 2010, 12, 1688 CrossRef CAS PubMed; (f) L.-L. Cao, Z.-S. Ye, G.-F. Jiang and Y.-G. Zhou, Adv. Synth. Catal., 2011, 353, 3352 CrossRef CAS; (g) J. Wang, S.-B. Zhou, D.-Z. Lin, X. Ding, H.-L. Jiang and H. Liu, Chem. Commun., 2011, 47, 8355 RSC; (h) M. Fochi, L. Gramigna, A. Mazzanti, S. Duce, S. Fantini, A. Palmieri, M. Petrini and L. Bernardi, Adv. Synth. Catal., 2012, 354, 1373 CrossRef CAS; (i) J.-Z. Huang, X. Wu and L.-Z. Gong, Adv. Synth. Catal., 2013, 355, 2531 CrossRef CAS; (j) S. Protti, A. Palmieri, M. Petrini, M. Fagnoni, R. Ballini and A. Albini, Adv. Synth. Catal., 2013, 355, 643 CrossRef CAS; (k) J. Luo, B. Wu, M.-W. Chen, G.-F. Jiang and Y.-G. Zhou, Org. Lett., 2014, 16, 2578 CrossRef CAS PubMed; (l) A. O. Kataja and G. Masson, Tetrahedron, 2014, 70, 8783 CrossRef CAS; (m) A. Palmieri, M. Petrini and R. R. Shaikh, Org. Biomol. Chem., 2010, 8, 1259 RSC.
- W.-G. Guo, B. Wu, X. Zhou, P. Chen, X. Wang, Y.-G. Zhou, Y. Liu and C. Li, Angew. Chem., Int. Ed., 2015, 54, 4522 CrossRef CAS PubMed.
- Water as the solvent in organic synthesis, for selected reviews, see: (a) C.-J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (b) C.-J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef CAS PubMed; (c) C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC; (d) C.-F. Pan and Z.-Y. Wang, Coord. Chem. Rev., 2008, 252, 736 CrossRef CAS; (e) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed; (f) M. O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC . For selected samples, see; (g) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7817 CrossRef; (h) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed; (i) Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless and M. G. Finn, J. Am. Chem. Soc., 2003, 125, 3192 CrossRef CAS PubMed; (j) T.-P. Loh and X.-R. Li, Angew. Chem., Int. Ed., 1998, 36, 980 CrossRef; (k) M.-Z. Lu, P. Lu, Y.-H. Xu and T.-P. Loh, Org. Lett., 2014, 16, 2614 CrossRef CAS PubMed; (l) Y.-W. Wei, D. Xue, Q. Lei, C. Wang and J.-L. Xiao, Green Chem., 2013, 15, 629 RSC; (m) C. Wang, C.-Q. Li, X.-F. Wu, A. Pettman and J.-L. Xiao, Angew. Chem., Int. Ed., 2009, 48, 6524 CrossRef CAS PubMed; (n) L. Zhong, Q. Gao, J.-B. Gao, J.-L. Xiao and C. Li, J. Catal., 2007, 250, 360 CrossRef CAS; (o) Q. Gao, Y. Liu, S.-M. Lu, J. Li and C. Li, Green Chem., 2011, 13, 1983 RSC; (p) B.-Y. Zhang, Z.-X. Jiang, X. Zhou, S.-M. Lu, J. Li, Y. Liu and C. Li, Angew. Chem., Int. Ed., 2012, 51, 13159 CrossRef CAS PubMed; (q) J. Li, Y.-M. Zhang, D.-F. Han, G.-Q. Jia, J.-B. Gao, L. Zhong and C. Li, Green Chem., 2008, 10, 608 RSC.
- For selected examples of the use of (thio)urea-based or squaramides as bifunctional organocatalysts, see: (a) B. Vakulya, S. Varga, A. Csámpai and T. Soós, Org. Lett., 2005, 7, 1967 CrossRef CAS PubMed; (b) S. H. McCooey and S. J. Connon, Angew. Chem., Int. Ed., 2005, 44, 6367 CrossRef CAS PubMed; (c) J. Wang, H. Li, L. Zu, W. Jiang, H. Xie, W. Duan and W. Wang, J. Am. Chem. Soc., 2006, 128, 12652 CrossRef CAS PubMed; (d) Y.-Q. Wang, J. Song, R. Hong, H. Li and L. Deng, J. Am. Chem. Soc., 2006, 128, 8156 CrossRef CAS PubMed; (e) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1424495. For ESI and crystallographic data in CIF or other electronic format, see DOI: 10.1039/c5cc07721d |
| This journal is © The Royal Society of Chemistry 2016 |