Tumor cell specific and lysosome-targeted delivery of nitric oxide for enhanced photodynamic therapy triggered by 808 nm near-infrared light†
Hui-Jing
Xiang
a,
Qiao
Deng
a,
Lu
An
b,
Min
Guo
a,
Shi-Ping
Yang
b and
Jin-Gang
Liu
*a
aKey Laboratory for Advanced Materials of MOE & Department of Chemistry, East China University of Science and Technology, Shanghai, 200237, P. R. China. E-mail: liujingang@ecust.edu.cn
bKey Laboratory of Resource Chemistry of MOE & Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai, 200234, P. R. China
First published on 15th October 2015
Abstract
A novel cancer cell lysosome-targetable multifunctional NO-delivery nanoplatform (Lyso-Ru-NO@FA@C-TiO2) (1) was developed. It selectively targets folate-receptor overexpressed cancer cells and specifically locates within the lysosome organelle to which NO and reactive oxygen species are simultaneously released upon 808 nm NIR light irradiation. The dual-targeted nanoplatform (1) demonstrated the highest anticancer efficacy compared with nontargeted counterparts under NIR light sensitization.
Nitric oxide (NO) not only plays critical roles in various physiological processes but has also been implicated in cancer biology.1 It has been shown to have both tumoricidal and tumor-promoting effects, depending on its timing, location, and concentration.1 At high concentrations (≥μM), NO acts as a potent anticancer agent, promoting apoptosis and inhibiting angiogenesis. The development of a NO-hybrid drug that combines NO with existing drugs such as photosensitizing agents employed in photodynamic therapy (PDT) may afford the advantage of adding the effects of NO to the benefits of drugs to overcome tumor cell resistance to conventional therapeutic agents.
In this context, a wide range of NO donors and their NO-delivery platforms have been developed, among which light-triggered NO-delivery platforms are found to be particularly attractive. This is so because light represents a suitable trigger for enabling both spatial and temporal control over NO release.2–5 However, most of the reported photoreactive NO-delivery platforms are effective only under UV or visible light irradiation, where the excitation wavelengths have suffered from the small depth of tissue penetration as well as cell photodamage from short wavelength light. Given its less-damaging and minimal absorbance by skin and tissues that allow for noninvasive deeper penetration, near-infrared (NIR) light is apparently more advantageous as a light trigger for NO delivery,6a especially for an 808 nm NIR light that has a minimized water overheating effect compared with other NIR wavelengths, e.g. 980 nm.6b Only a few limited examples have demonstrated NIR light-triggered NO release.4,7
Lysosomes are important subcellular acidic organelles for cellular homeostasis, and lysosomal dysfunction is responsible for several diseases. Progressive evidence has suggested that the lysosomal death pathway could markedly contribute to the programmed cell death sensitivity of cancer cells.8 Thus, the development of a NO-hybrid drug that specifically delivers NO to lysosomes will render the drug with maximal therapeutic efficacy and minimal side effects.
Herein, we report a novel cancer cell lysosome-targetable multifunctional NO-delivery nanoplatform (Lyso-Ru-NO@FA@C-TiO2) (1). This nanoplatform selectively targets folate-receptor (FR) overexpressed cancer cells and specifically locates within the lysosome organelle to which NO and reactive oxygen species (ROS) are simultaneously delivered upon 808 nm NIR light irradiation with mild power intensity (200–600 mW cm−2) (Fig. 1).9 This represents the first example of cancer cell lysosome-specific codelivery of NO and ROS on demand using controlled NIR light.
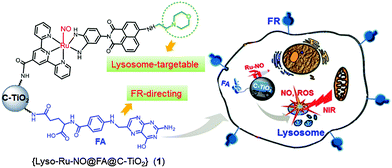 | ||
| Fig. 1 Schematic representation of nanoplatform (1) and the directed-attack of cancer cell lysosomes by NO and ROS under NIR light irradiation. | ||
The NO-delivery nanoplatform (1) is composed of a ruthenium nitrosyl donor, [Ru(tpyCOOH)(Lyso-NINO)(NO)] (PF6)3, (Lyso-Ru-NO), a cancer cell directing group of folic acid (FA), and a carrier of biocompatible carbon-doped titanium dioxide nanoparticles (C-TiO2 NPs). The designed novel ruthenium nitrosyl Lyso-Ru-NO contains a ligand of Lyso-NINO wherein a morpholine moiety serves as a well-known lysosomal targeting group.10 The FA group is used as a tumor-targeting component on the basis of its high affinity to FR, which is often overexpressed on the surface of a wide range of human cancerous cells (e.g., HeLa and KB cell lines).11 Given their unique photocatalytic properties, good biocompatibility, and low cytotoxicity, TiO2 NPs have been well documented in the biomedical applications of PDT and drug delivery.12 Carbon doping may generate new hybrid states in the band gap of TiO2, which render C-TiO2 NPs with significant absorbance in long wavelength regions.13 Thus, we employed C-TiO2 NPs as carriers for NIR light-triggered NO delivery, expecting that at the same time C-TiO2 NPs may work as efficient photosensitizers to generate cytotoxic ROS under NIR light illumination.
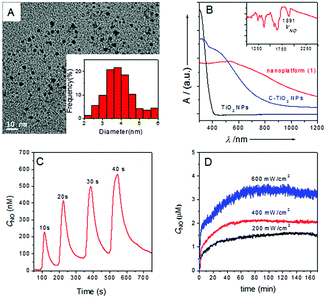
The procedures for the preparation of the ruthenium nitrosyl Lyso-Ru-NO and its corresponding NO-delivery nanoplatform (1) are described in the ESI.† The morphology of nanoplatform (1) was examined using transmission electron microscopy (TEM), and displayed a spherical morphology with diameters of about 4 nm (Fig. 2A). Its surface chemical composition was then analyzed using X-ray photoelectron spectroscopy (Fig. S1, ESI†). The amount of Lyso-Ru-NO incorporated onto the surface of C-TiO2 NPs was determined to be 6.64 wt% Ru, which corresponds to 0.65 μmol NO per milligram of the nanoplatform.
The diffuse reflectance UV/vis-NIR absorption spectrum of nanoplatform (1) showed significant enhancement of light absorption at wavelengths in the range 600–1200 nm compared to C-TiO2 NPs, suggesting that the grafting of Lyso-Ru-NO onto the surface of C-TiO2 NPs further intensified its absorbance in the long wavelength region (Fig. 2B). The FTIR spectrum of nanoplatform (1) displayed a νNO peak at 1891 cm−1 (Fig. 2B inset), which slightly shifted to higher energy compared to that of uncoupled ruthenium nitrosyl Lyso-Ru-NO (1898 cm−1, Fig. S2B, ESI†). Nanoplatform (1) exhibited a photoluminescence peak centered at 468 nm (Fig. S3, ESI†) when excited at a wavelength of 280 nm. The blue fluorescence characteristic makes it possible to track the nanoplatform in cells. The X-ray diffraction (XRD) spectra of nanoplatform (1) displayed patterns similar to those of C-TiO2 NPs with (101), (004), (200), and (105) reflections that are characteristic of anatase TiO2 (Fig. S4, ESI†). This result indicates that the surface incorporation of ruthenium nitrosyl does not alter the C-TiO2 NP structure.
NIR light-triggered NO release from nanoplatform (1) was probed using an ultrasensitive NO electrode, which directly detects NO using an amperometric technique. As shown in Fig. 2C, an incremental NO flux was obtained when the suspension saline solution of nanoplatform (1) was irradiated by an 808 nm NIR laser upon the duration time being increased from 10 s to 40 s under a very mild power intensity (200 mW cm−2).9 The delivered NO flux was readily tuned by the laser pulse through variation of both pulse duration time and pulse intensity (Fig. S5A and B, ESI†). Moreover, the off-and-on behavior of NO release from nanoplatform (1) revealed constant “zig-zag” release profiles (Fig. S5C, ESI†), which indicated a fast response of nanoplatform (1) to the applied NIR laser and also suggested that nanoplatform (1) has no light fatigue effect under repeated off-and-on laser irradiation. A 1.0 mg mL−1 of the nanoplatform (1) sample suspended in a saline solution can produce a steady level of NO at higher than micromolar concentrations (≥μM) under constant 808 nm NIR light illumination even for light intensities as low as 200 mW cm−2 (Fig. 2D). Therefore, these results unambiguously demonstrate that efficient NO release from nanoplatform (1) can be achieved under the total control of an 808 nm NIR laser with mild power intensity. The quantum yield (ΦNO) for NO release from nanoplatform (1) under 808 nm light irradiation was measured to be 0.0174 ± 0.002 mol Einstein−1. In addition, nanoplatform (1) showed good stability under various conditions when kept in the dark, even in acidic solutions (Fig. S6, ESI†). Photo-triggered electron transfer and energy transfer from the C-TiO2 to the NO donor and the surrounding O2 are believed to be responsible for the NO release and ROS generation from the nanoplatform (1) under 808 nm laser irradiation (Fig. S7, ESI†).
The generation of extracellular ROS was then verified using the ROS fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH DA).14 The fluorescence intensity of the solution of DCFH DA steadily increased in the presence of nanoplatform (1) under 808 nm NIR light irradiation (Fig. S5D, ESI†), which reached an 8.7-fold increase relative to that obtained in the absence of light irradiation. The singlet oxygen quantum yields (ΦΔ) under 808 nm light irradiation were determined to be in the range 0.23–0.28 with pH from 8.0 to 4.0 (ESI†). It is noteworthy that under similar experimental conditions, the fluorescence intensity increased only 2.1-fold for the solution of DCFH DA treated with C-TiO2 NPs under 808 nm NIR light irradiation (Fig. S5E, ESI†). These results indicated that nanoplatform (1) is more efficient than C-TiO2 NPs for generating ROS when sensitized by NIR light.
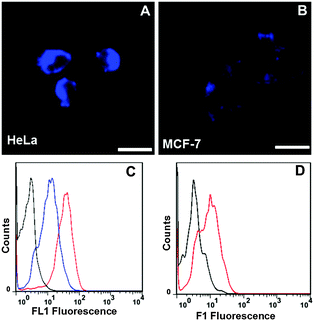
To gain more insight into the nanoplatform (1) interaction with cells, we investigated whether or not the FA moiety guided the nanoplatform preferentially over FR-positive tumor cells. FR-positive HeLa cells and FR-negative MCF-7 cells were then incubated with nanoplatform (1), respectively. As shown in Fig. 3A, the HeLa cells exhibited a strong fluorescence within 2 h of incubation with nanoplatform (1), whereas the MCF-7 cells showed a very weak fluorescence under the same experimental conditions (Fig. 3B), suggesting the selective accumulation of nanoplatform (1) in FR-positive cancer cells. Similar results were obtained using flow cytometry (FCM) analysis. HeLa cells treated with nanoplatform (1) yielded far more intense fluorescence compared with MCF-7 cells (Fig. 3C and D). In addition, competition experiments with free FA were carried out, wherein nanoplatform (1) (50 μg mL−1) and free FA (50 μg mL−1) were incubated together with HeLa cells for 2 h. The results of FCM analysis revealed markedly decreased fluorescence intensity (Fig. 3C). As a result, it can be concluded that nanoplatform (1) entering into cells was guided by the interactions of its FA moiety with FR on the surface of tumor cells, possibly via receptor-mediated endocytosis.
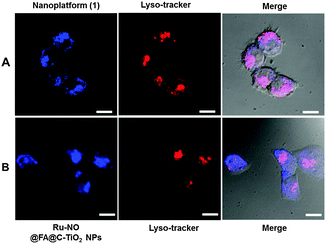
To further explore the intracellular localization, HeLa cells were treated with nanoplatform (1) together with Lysotracker Red, a commercial lysosome tracker. The intracellular localization of nanoplatform (1) and Lysotracker Red can then be visualized using confocal laser fluorescence microscopy under different FL channels. Nanoplatform (1) is readily visualized by its blue fluorescence, and Lysotracker Red is visualized by its red fluorescence. The merged image shows the colour magenta (Fig. 4A) with a high Pearson’s co-localization coefficient (0.84). This result clearly indicates the internalized nanoplatform (1) specifically located in the lysosome organelle. A control experiment with another nanoplatform (Ru-NO@FA@C-TiO2) was carried out, where the ruthenium nitrosyl was devoid of the lysosome-targeting morpholine moiety. As shown in Fig. 4B, the nanoplatform (Ru-NO@FA@C-TiO2) is randomly distributed inside the cells with Pearson’s co-localization coefficient of −0.06, suggesting no specific lysosomal localization. These observations evidently signify the important role of the morpholine group in nanoplatform (1) that render it to specifically target lysosomes.
The in vitro generation of NO was then examined using the NO-sensitive fluorescent probe 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA).15 HeLa cells were incubated with nanoplatform (1) (50 μg mL−1) for 4 h and then treated with the DAF-FM DA probe for 30 min. In the absence of light irradiation, only very weak green fluorescence from the probe itself was observed, whereas bright green fluorescence was noticed once the 808 nm NIR laser was applied (Fig. S8, ESI†), indicating quick NO release from nanoplatform (1) within the cells.
Intracellular ROS generation was monitored via the ROS fluorescent probe DCFH-DA. After incubation with nanoplatform (1) in the dark, HeLa cells were irradiated with an 808 nm laser. The intracellular fluorescence intensity, as analyzed by FCM, is generally proportional to the amounts of generated ROS. The fluorescence intensity steadily increased with the time of NIR light illumination (Fig. S9, ESI†), suggesting that nanoplatform (1) could serve in vitro as an efficient photosensitizer under NIR light irradiation for photodynamic therapy.
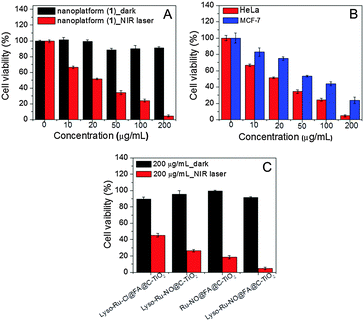
Subsequently, an MTT assay was performed to evaluate the anticancer efficiency of nanoplatform (1) under NIR light irradiation. When treated with nanoplatform (1) of various concentrations (10–200 μg mL−1) in the dark, the cell viability of both HeLa and MCF-7 cells remained above 85%, suggesting a good biocompatibility of the nanoplatform (Fig. S10 and S11, ESI†) while under the irradiation of an 808 nm NIR laser (600 mW cm−2, 10 min), the HeLa cell viability remarkably decreased, which showed drug-dosage dependence (Fig. 5A). The half maximal inhibitory concentration (IC50) of nanoplatform (1) toward HeLa cells under 808 nm NIR light sensitization was about 20 μg mL−1. It is interesting to note that the cell viability of FR-negative MCF-7 cells was much higher than that of FR-positive HeLa cells under similar experimental conditions (Fig. 5B). In addition, the cell viability was also higher when it was treated with the control nanoplatform (Lyso-Ru-NO@C-TiO2) without an FA group (Fig. 5C). These results indicate the beneficial anticancer effect of the FA-directing group that accounts for higher nanoplatform (1) accumulation in the targeted cancer cells.
Furthermore, in the absence of the lysosome-targetable morpholine group, the control nanoplatform (Ru-NO@FA@C-TiO2)-treated HeLa cells also showed higher viability than nanoplatform (1) under 808 nm NIR light irradiation (Fig. 5C), suggesting that the increased anticancer efficiency was because of the intracellular lysosome-localization of nanoplatform (1). Taken together, these results clearly indicate that the dual-targeted nanoplatform (1) demonstrated the maximum anticancer effect.
To differentiate the therapeutic effect exerted by NO and ROS as observed for nanoplatform (1), a further control experiment with a nanoplatform (Lyso-Ru-Cl@FA@C-TiO2) without coordinated NO was tested for cytotoxicity. With the dosage of 200 μg mL−1 of nanoplatforms under NIR light sensitization, the viability of HeLa cells treated with (Lyso-Ru-Cl@FA@C-TiO2) (∼46%) was markedly higher than that of HeLa cells treated with nanoplatform (1) (∼5%, Fig. 5C), thus confirming the synergistic cytotoxicity effects of both released NO and ROS. The main cell death mechanism, as revealed by FCM analysis using Annexin V-FITC/propidium iodide (PI) double-staining assay, was suggested to be early apoptosis (Fig. S12, ESI†).
In summary, a novel multifunctional nanoplatform (1) (Lyso-Ru-NO@FA@C-TiO2) with cellular and subcellular dual-targetable delivery of NO and ROS under 808 nm NIR light irradiation was demonstrated for the first time. The incorporation of FA- and morpholine-directing groups onto the nanoplatform (1) renders it capable of targeting FR-positive cancer cell lines and specific accumulation in the subcellular lysosomal organelles. Furthermore, the inherent blue fluorescence of nanoplatform (1) enables it to be readily tracked during its cellular internalization and subcellular distribution. The cytotoxicity assay manifested that the dual-targeted nanoplatform (1) has the highest anticancer efficacy when compared with the efficacy of its nontargeted counterparts under NIR light sensitization. Therefore, this multifunctional platform garnered cancer cell selectivity, subcellular lysosome-targetable, mild NIR light (808 nm, 200–600 mW cm−2)-triggered NO and ROS release and cell imaging functionalities into one system. Such a novel NO-delivery nanoplatform adds additional NO beneficial effects to traditional PDT and would have new implications for lysosome-targeted and NO-mediated multimodal phototherapy.
This study was financially supported by the NSF of China (21271072, 21571062), the Program for Professor of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning, and sponsored by the Shanghai Pujiang Program (13PJ1401900).
Notes and references
- A. R. Butler and R. Nicholson, Life, Death and Nitric Oxide, the Royal Society of Chemistry, Cambridge, 2003 Search PubMed.
- (a) M. J. Rose and P. K. Mascharak, Coord. Chem. Rev., 2008, 252, 2093 CrossRef CAS PubMed; (b) N. L. Fry and P. K. Mascharak, Acc. Chem. Res., 2011, 44, 289 CrossRef CAS PubMed; (c) B. J. Heilman, J. St. John, S. R. J. Oliver and P. K. Mascharak, J. Am. Chem. Soc., 2012, 134, 11573 CrossRef CAS PubMed.
- (a) S. Sortino, J. Mater. Chem., 2012, 22, 301 RSC; (b) S. Sortino, Chem. Soc. Rev., 2010, 39, 2903 RSC; (c) J. Kim, G. Saravanakumar, H. W. Choi, D. Park and W. J. Kim, J. Mater. Chem. B, 2014, 2, 341 RSC.
- (a) P. C. Ford, Nitric oxide, 2013, 34, 56 CrossRef CAS PubMed; (b) J. V. Garcia, J. Yang, D. Shen, C. Yao, X. Li, R. Wang, G. D. Stucky, D. Zhao, P. C. Ford and F. Zhang, Small, 2012, 8, 3800 CrossRef CAS PubMed; (c) P. T. Burks, J. V. Garcia, R. GonzalezIrias, J. T. Tillman, M. Niu, A. A. Mikhailovsky, J. Zhang, F. Zhang and P. C. Ford, J. Am. Chem. Soc., 2013, 135, 18145 CrossRef CAS PubMed.
- H.-J. Xiang, L. An, W.-W. Tang, S.-P. Yang and J.-G. Liu, Chem. Commun., 2015, 51, 2555 RSC.
- (a) J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626 CrossRef CAS PubMed; (b) X. Xie and X. Liu, Nat. Mater., 2012, 11, 842 CrossRef CAS PubMed.
- (a) L. Tan, A. Wan, X. Zhu and H. Li, Chem. Commun., 2014, 50, 5725 RSC; (b) X. Zhang, G. Tian, W. Yin, L. Wang, X. Zheng, L. Yan, J. Li, H. Su, C. Chen, Z. Gu and Y. Zhao, Adv. Funct. Mater., 2015, 25, 3049 CrossRef CAS.
- N. Fehrenbacher and M. Jaattela, Cancer Res., 2005, 65, 2993 CAS.
- Using two-photon excitation or upconversion nanoparticles with NIR light to activate the NO-releasing precursors, the excitation power density required was much higher. See ref. 4 and 7.
- H. Yu, Y. Xiao and L. Jin, J. Am. Chem. Soc., 2012, 134, 17486 CrossRef CAS PubMed.
- N. Parker, M. J. Turk, E. Westrick, J. D. Lewis, P. S. Low and C. P. Leamon, Anal. Biochem., 2005, 338, 284 CrossRef CAS PubMed.
- (a) Z. Hou, Y. Zhang, K. Deng, Y. Chen, X. Li, X. Deng, Z. Cheng, H. Lian, C. Li and J. Lin, ACS Nano, 2015, 9, 2584 CrossRef CAS PubMed; (b) C. Ratanatawanate, A. Chyao and K. J. Balkus Jr., J. Am. Chem. Soc., 2011, 133, 3492 CrossRef CAS PubMed.
- J. Zhong, F. Chen and J. Zhang, J. Phys. Chem. C, 2010, 114, 933 CAS.
- C. Zhu, Q. Yang, L. Liu, F. Lv, S. Li, G. Yang and S. Wang, Adv. Mater., 2011, 23, 4805 CrossRef CAS PubMed.
- H. Kojima, Y. Urano, K. Kikuchi, T. Higuchi, Y. Hirata and T. Nagano, Angew. Chem., Int. Ed., 1999, 38, 3209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures. See DOI: 10.1039/c5cc07006f |
| This journal is © The Royal Society of Chemistry 2016 |