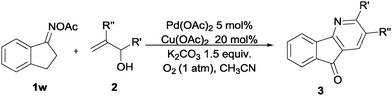
Palladium-catalyzed Heck-type reaction of oximes with allylic alcohols: synthesis of pyridines and azafluorenones†
Meifang
Zheng
,
Pengquan
Chen
,
Wanqing
Wu
and
Huanfeng
Jiang
*
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: jianghf@scut.edu.cn; Fax: +86 20-87112906; Tel: +86 20-87112906
First published on 16th October 2015
Abstract
We describe herein a palladium-catalyzed Heck-type reaction of O-acetyl ketoximes and allylic alcohols to synthesise pyridines. This protocol allows the robust synthesis of pyridines and azafluorenones in good to excellent yields with tolerance of various functional groups under mild conditions. The reaction is supposed to go through an oxidative addition of oximes to palladium(0) complexes, generating an alkylideneamino-palladium(II) species, which is utilized as a key intermediate to capture the nonbiased alkenes for carbon–carbon bond formation.
N-heterocycles are some of the most important building blocks in organic chemistry.1 Among them, pyridines are one of the most significant building blocks, which not only exist extensively in pharmaceuticals, agrochemicals, and functional materials, but also serve as useful synthetic intermediates in modern organic synthesis.2 Considering the large spectrum of fascinating applications, the synthesis of pyridine derivatives has long been an area of intense interest, and a number of different methods have been devised for their preparation.3 However, challenges still remain in the scope of group tolerance and equivalent usage of catalysts. Therefore, the development of methodologies for a more direct and efficient access to different substituted and specifically functionalized frameworks is always a great challenge in modern synthetic organic chemistry.
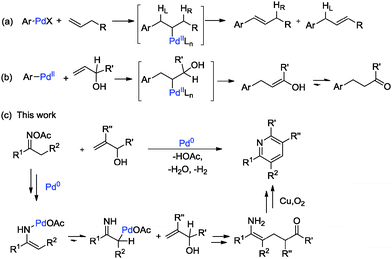
The development of catalytic C–C bond-forming reactions remains one of the main challenges in organic chemistry.4 Among the transition metal-catalyzed reactions known to form C–C bonds, the palladium-catalyzed Mizoroki–Heck oxidative coupling stands out as one of the most valuable synthetic tools.5 Since its first report in the 1970s, lots of efforts have been devoted to its utilization, and it is now fully recognized as one of the most significant methods for carbon–carbon bond formations.6 Given that most of the oxidative Heck reactions were limited to active alkenes or styrenes due to the poor reactivity of unactivated olefins7 and the dilemma of selective β-H elimination8 (Scheme 1a), therefore, the transition metal-catalyzed olefination of nonbiased allylic alcohols, without prior functionalization, is even more environmentally friendly and versatile (Scheme 1b).9 According to previous reports, oximes and their derivatives can be used as “internal oxidants” towards the transition metal-catalyzed oxidative C–H functionalization and cleavage of the N–O bond by the participation of transition metals to form active alkylideneaminopalladium(II) species, which tautomerized to the key iminylpalladium(II) intermediates, making the following alkylpalladium-catalyzed oxidative Heck-type reaction possible (Scheme 1c).10,11 In this regard, based on our previous work on oximes12 and allylic alcohols as alkyl ketone resources,13 we design a possible [3+3] type cyclization with these two substrates and suppose the reaction to go through a palladium-catalyzed Heck-type coupling of oximes with alkenes, with high efficiency under mild reaction conditions.
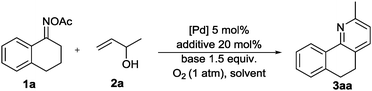
With the ability of palladium to reduce oxime N–O bonds, we chose 3,4-dihydronaphthalen-1(2H)-one O-acetyl oxime (1a) and but-3-en-2-ol (2a) as model substrates and screened different additives and other reaction conditions (Table 1). To our delight, the desired product 3aa could be detected in 56% yield when the model reaction was conducted in CH3CN with CuBr2 as an additive in the presence of the Pd(OAc)2 catalyst under a dioxygen atmosphere at 80 °C for 24 h (entry 1). The screening of different additives, including Cu(OAc)2, KI, and AgNO3, revealed that 3aa could also be formed in lower yields (entries 2–4). And Cu(OAc)2 was found to be the best additive and the yield of 3aa could reach 87% (entry 2). However, with other additives, such as LiBr, NH4Br, Bu4NBr and AgOAc, only a trace amount of 3aa was detected (entries 5–8). The model reaction was then tested using other palladium catalytic systems, and Pd(OAc)2 was found to be the most suitable catalyst system for the target reaction (entries 9–11). No reaction was observed in the absence of a palladium catalyst (entry 12), while in the absence of Cu(OAc)2, the reaction could give 10% yield of product 3aa (entry 13). The yield was unsatisfactory with the decrease of the reaction temperature (entry 14), while raising the temperature to 100 °C improved the yield to 91% (entry 15). When the reaction was carried out in N2 atmosphere, only a trace amount of 3aa was detected by GC-MS (entry 17).
| Entrya | Catalyst | Additive | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: unless otherwise noted, the reaction was carried out using 1a (0.2 mmol), 2a (0.4 mmol), the Pd catalyst (5 mol%), an additive (20 mol%) and a base (1.5 equiv.) in a solvent (1 mL) at 80 °C for 24 h. b Yield determined by GC-MS using dodecane as an internal standard, and isolated yield in the parentheses. c The reaction was carried out at 60 °C. d The reaction was carried out at 100 °C. e The reaction was carried out in the open air. f The reaction was carried out in N2 atmosphere. | |||||
| 1 | Pd(OAc)2 | CuBr2 | K2CO3 | CH3CN | 56 |
| 2 | Pd(OAc)2 | Cu(OAc)2 | K2CO3 | CH3CN | 87 (87) |
| 3 | Pd(OAc)2 | KI | K2CO3 | CH3CN | 10 |
| 4 | Pd(OAc)2 | AgNO3 | K2CO3 | CH3CN | 25 |
| 5 | Pd(OAc)2 | LiBr | K2CO3 | CH3CN | Trace |
| 6 | Pd(OAc)2 | NH4Br | K2CO3 | CH3CN | Trace |
| 7 | Pd(OAc)2 | Bu4NBr | K2CO3 | CH3CN | Trace |
| 8 | Pd(OAc)2 | AgOAc | K2CO3 | CH3CN | Trace |
| 9 | PdCl2 | Cu(OAc)2 | K2CO3 | CH3CN | 57 |
| 10 | Pd(OTf)2 | Cu(OAc)2 | K2CO3 | CH3CN | 63 |
| 11 | Pd2(dba)3 | Cu(OAc)2 | K2CO3 | CH3CN | Trace |
| 12 | — | Cu(OAc)2 | K2CO3 | CH3CN | n.d. |
| 13 | Pd(OAc)2 | — | K2CO3 | CH3CN | 10 |
| 14c | Pd(OAc)2 | Cu(OAc)2 | K2CO3 | CH3CN | 35 |
| 15d | Pd(OAc)2 | Cu(OAc)2 | K2CO3 | CH3CN | 91 |
| 16e | Pd(OAc)2 | Cu(OAc)2 | K2CO3 | CH3CN | 78 |
| 17f | Pd(OAc)2 | Cu(OAc)2 | K2CO3 | CH3CN | Trace |
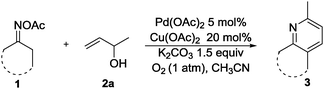
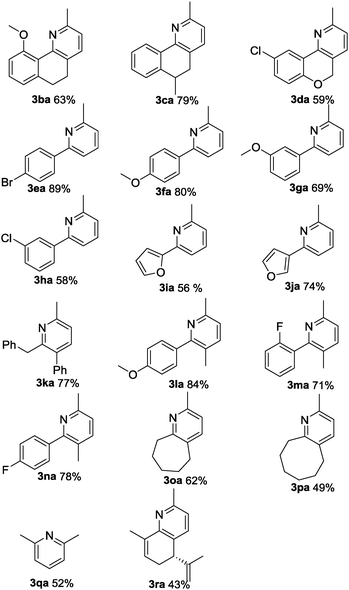
With the optimal reaction conditions in hand, we subsequently explored the reaction scope (Table 2). The oxime substrates derived from other aryl ketones such as tetralones, propiophenones and 2-phenylacetophenones could afford the products 3aa–3na in good to excellent yields. Besides, oximes derived from aliphatic ketones afforded the pyridine derivatives 3oa–3ra in moderate to good yields. To be specific, chroman-4-one oxime could afford 3da in 59% yield. The formation of 3da and 3ea was inert under these conditions, indicating that the palladium catalyst preferentially reacts with the N–O bond of the oxime acetate over the carbon–halogen bonds. Other aryl functionalized amides could also undergo the pathway well. The 2-furan-substituted and 3-furan-substituted oximes could be tolerated in this transformation, generating 3ia and 3ja in 56% and 74% yields, respectively. It is noteworthy that alkyl-substituted oximes were also suitable substrates in this reaction with good yields (3oa to 3ra). For example, propone oximes that contained short alkyl chains gave the product 2,6-dimethylpyrudine in moderate yield (3qa). Notably, L(−)-carvone derived oximes could be transformed into 3ra in 43% yield.
| a Reaction conditions: unless otherwise noted, the reaction was carried out using 1 (0.5 mmol), 2a (1.0 mmol), Pd(OAc)2 (5 mol%), Cu(OAc)2 (20 mol%) and K2CO3 (1.5 equiv.) in CH3CN (1 mL) at 80 °C for 16–24 h. |
|---|
|
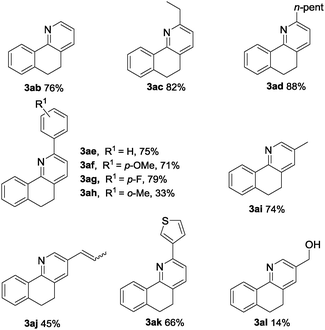
We next focused our attention on the scope and generality of the olefin substrates in the oxidative coupling with oxime 1a. This transformation displayed high functional group tolerance and proved to be a quite general method. Both aliphatic and aromatic allylic alcohols could undergo the protocol with good to excellent yields (Table 3). Alkyl-substituted allyl alcohols were found to be suitable substrates for this transformation and gave the desired products in good yields (3aa to 3ad). Even the bulky aromatic allylic alcohols with methyl, methoxyl and fluoro on aryl rings all gave good to excellent yields of the corresponding pyridines (3af–3ah). To be specific, 3ah could be obtained in 35% yield, which implied that the steric hindrance had little effect on this transformation. And the substrate with aryl substitution of 1-(thiophen-3-yl)prop-2-en-1-ol underwent the Heck reaction smoothly and gave the corresponding product 3ak in 66% yield. As for the application of hexa-1,5-dien-3-ol in this reaction, the hydrogen immigration product 3aj was obtained in 45% yield, which may further support the role of palladium in the β-H elimination. Besides, with the application of 2-methylenepropane-1,3-diol as the coupling reagent, the yield of the desired product 3al sharply decreased to 14% and 50% yield of 3ai was obtained.14
| a Reaction conditions: unless otherwise noted, the reaction was carried out using 1a (0.5 mmol), 2 (1.0 mmol), Pd(OAc)2 (5 mol%), Cu(OAc)2 (20 mol%) and K2CO3 (1.5 equiv.) in CH3CN (1 mL) at 80 °C for 16–24 h. |
|---|
|
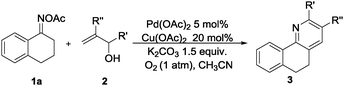
Furthermore, using 1-indanone oxime as a substrate, different azafluorenones could be obtained in this system (Table 4).15 For example, the reaction of 2,3-dihydro-1H-inden-1-one O-acetyl oxime (1w) afforded the corresponding 4-azafluorenones in good yield. Pleasingly, when 5,6-dihydro-7H-cyclopenta[b]pyridin-7-one O-acetyl oxime (1x) was applied, 39% yield of 4,5-diazafluorenone was obtained.16
| Entry | R′, R′′ | Oximes | Yield (%) |
|---|---|---|---|
| a Reaction conditions: unless otherwise noted, the reaction was carried out using 1 (0.5 mmol), 2 (1.0 mmol), Pd(OAc)2 (5 mol%), Cu(OAc)2 (20 mol%) and K2CO3 (1.5 equiv.) in CH3CN (1 mL) at 80 °C for 16–24 h. | |||
| 1 | R′ = Me, R′′ = H |
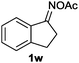
|
45 |
| 2 | R′ = H, R′′ = H | 67 | |
| 3 | R′ = H, R′′ = Me | 47 | |
| 4 | R′ = H, R′′ = H |
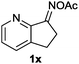
|
39 |
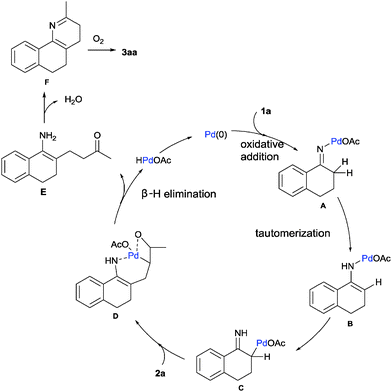
Based on the experimental results17 and previous reports, a plausible mechanism is proposed in Scheme 2. Firstly, the oxidative addition of the oxime N–O bond to Pd(0) species easily led to the palladium enamide intermediate A. Subsequent tautomerization affords the enamine-derived amido-Pd(II) species B.11 Further isomerization of this species results in the conversion of this N-bound enamido-Pd(II) B species to its C-bound isomer C.18 Followed by Mizoroki–Heck reaction of allylic alcohol 2a, insertion into the carbon–palladium bond of alkylpalladium species C forms an intermediate species D.19 Then, intermediate D undergoes β-H elimination to give intermediate E and regenerates the active palladium catalyst.14 The intramolecular condensation of the amine and ketone moieties of intermediate E forms F. Finally, the dehydrative oxidation gives the pyridine product 3aa.20 An alternative mechanism is shown in the ESI.†
In summary, we have successfully developed a palladium-catalyzed strategy to construct pyridines from O-acetyl oximes and nonbiased alkenes. This protocol provides a possible pathway for the insertion of alkenes into oximes. With the operational simplicity, efficiency and functional group compatibility, this protocol will expand the scope of pyridine derivatives. Furthermore, the present method also enables the direct construction of a diverse array of azafluorenone cores, which may find applications in natural product synthesis and medicinal chemistry.
The authors thank the National Natural Science Foundation of China (21172076, 21202046 and 21420102003), the Fundamental Research Funds for the Central Universities (2014ZP0004), and the Doctoral Fund of the Department of Education of Guangdong (sybzzxm201310) for financial support.
Notes and references
- For recent reviews, (a) J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642 CrossRef CAS PubMed; (b) N. R. Candeias, C. Brancol, P. M. P. Gois, C. A. M. Afonso and A. F. Trindade, Chem. Rev., 2009, 109, 2703 CrossRef CAS PubMed; (c) M. T. Aranda, P. Perez and R. Gonzalez, Curr. Org. Synth., 2009, 6, 325 CrossRef CAS; (d) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed; (e) B. Alcaide, P. Almendros and C. Aragoncillo, Chem. Rev., 2007, 107, 4437 CrossRef CAS PubMed; (f) X.-F. Wu and M. Beller, Heterocycles from Double-Functionalized Arenes: Transition Metal Catalyzed Coupling Reactions, RSC, 2015 Search PubMed; (g) X.-F. Wu and M. Beller, Economic Synthesis of Heterocycles: Zinc, Iron, Copper, Cobalt, Manganese and Nickel Catalysts, RSC, 2014 Search PubMed; (h) X.-F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113, 1 CrossRef CAS PubMed.
- (a) C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829 CrossRef CAS PubMed; (b) S. Zheng, Q. Zhong, M. Mottamal, Q. Zhang, C. Zhang, E. LeMelle, H. McFerrin and G. Wang, J. Med. Chem., 2014, 57, 3369 CrossRef CAS PubMed; (c) J. Bonin, C. Costentin, M. Robert and J.-M. Savéant, Org. Biomol. Chem., 2011, 9, 4064 RSC; (d) D. M. Stout and A. I. Meyers, Chem. Rev., 1982, 82, 223 CrossRef CAS; (e) C.-P. Cao, W. Lin, M.-H. Hu, Z.-B. Huang and D.-Q. Shi, Chem. Commun., 2013, 49, 6983 RSC.
- For examples of pyridine synthesis, see: (a) Y. Wei and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 3756 CrossRef CAS PubMed; (b) Z.-H. Ren, Z.-Y. Zhang, B.-Q. Yang, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2011, 13, 5394 CrossRef CAS PubMed; (c) K. Wu, Z. Huang, C. Liu, H. Zhang and A. Lei, Chem. Commun., 2015, 51, 2286 RSC; (d) L.-Y. Xi, R.-Y. Zhang, S. Liang, S.-Y. Chen and X.-Q. Yu, Org. Lett., 2014, 16, 5269 CrossRef CAS PubMed; (e) H. Huang, X. Ji, W. Wu, L. Huang and H. Jiang, J. Org. Chem., 2013, 78, 3774 CrossRef CAS PubMed; (f) K. Parthasarathy, M. Jeganmohan and C.-H. Cheng, Org. Lett., 2008, 10, 325 CrossRef CAS PubMed.
- For reviews, see: (a) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (b) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed; (c) J. Hassan, M. Svignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed; (d) J. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed; (e) C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (f) W. Wu and H. Jiang, Acc. Chem. Res., 2014, 47, 2483 CrossRef CAS PubMed.
- (a) R. F. Heck, Acc. Chem. Res., 1979, 12, 146 CrossRef CAS; (b) R. F. Heck, Org. React., 1982, 27, 345 CAS; (c) R. F. Heck, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 4, p. 833 Search PubMed; (d) M. Kitamura and K. Narasaka, The Chemical Record., 2002, 2, 268 CrossRef CAS PubMed; (e) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed.
- For recent selected examples of Heck reactions, see: (a) Y. Izawa, C. Zheng and S. S. Stahl, Angew. Chem., Int. Ed., 2013, 52, 3672 CrossRef CAS PubMed; (b) S. Arai, T. Sato, Y. Koike, M. Hayashi and A. Nishida, Angew. Chem., Int. Ed., 2009, 48, 4528 CrossRef CAS PubMed; (c) C. Pi, Y. Li, X. L. Cui, H. Zhang, Y. Han and Y. Wu, Chem. Sci., 2013, 4, 2675 RSC; (d) E. Alza, L. Laraia, B. M. Ibbeson, S. Collins, W. R. J. D. Galloway, J. E. Stokes, A. R. Venkitaraman and D. R. Spring, Chem. Sci., 2015, 6, 390 RSC; (e) C. M. McMahon and E. J. Alexanian, Angew. Chem. Int. Ed., 2014, 53, 5974 CrossRef CAS PubMed; (f) S. Ma and Z. Gu, Angew. Chem. Int. Ed., 2005, 44, 7512 CrossRef CAS PubMed.
- (a) K. H. Jensen, T. P. Pathak and Y. Zhang, J. Am. Chem. Soc., 2009, 131, 17074 CrossRef CAS PubMed; (b) W. E. Werner, J. Am. Chem. Soc., 2010, 132, 13981 CrossRef PubMed; (c) R. J. DeLuca and M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 11454 CrossRef CAS PubMed; (d) W. Erik, E. W. Werner, T.-S. Mei, A. J. Burckle and M. S. Sigman, Science, 2012, 338, 1455 CrossRef PubMed; (e) X. Tong, M. Beller and M. K. Tse, J. Am. Chem. Soc., 2007, 129, 4906 CrossRef CAS PubMed; (f) G. Yue, K. Lei, H. Hirao and J. Zhou, Chem. Sci., 2013, 4, 2675 RSC; (g) M. Kitamura, D. Kudo and K. Narasaka, Arkivoc, 2006, 148 CAS.
- (a) M. S. Chen and M. C. White, J. Am. Chem. Soc., 2004, 126, 1346 CrossRef CAS PubMed; (b) A. Kubota, M. H. Emmert and M. S. Sanford, Org. Lett., 2012, 14, 1760 CrossRef CAS PubMed; (c) L. Qin, X. Ren, Y. Lu, Y. Li and J. Zhou, Angew. Chem. Int. Ed., 2012, 51, 5915 CrossRef CAS PubMed.
- (a) M. Chen, J. Wang, Z. Chai, C. You and A. Lei, Adv. Synth. Catal., 2012, 354, 341 CrossRef CAS PubMed; (b) M. Vellakkaran, M. M. S. Andappan and N. Kommu, Eur. J. Org. Chem., 2012, 4694 CrossRef CAS PubMed; (c) Z. Shi, M. Boultadakis-Arapinis and F. Glorius, Chem. Commun., 2013, 49, 6489 RSC.
- For recent reviews of Pd-catalysis using the N–O bond as an internal oxidant, see: (a) M. Kitamura and K. Narasaka, Chem. Rec., 2002, 2, 268 CrossRef CAS PubMed; (b) K. Narasaka and M. Kitamura, Eur. J. Org. Chem., 2005, 4505 CrossRef CAS PubMed; (c) H. Huang, X. Ji, W. Wu and H. Jiang, Chem. Soc. Rev., 2015, 44, 1155 RSC.
- For examples of Pd-catalysis using the N–O bond as an internal oxidant, see: (a) J. Wu, X. Cui, L. Chen, G. Jiang and Y. Wu, J. Am. Chem. Soc., 2009, 131, 13888 CrossRef CAS PubMed; (b) W. P. Hong, A. V. Iosub and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 13664 CrossRef CAS PubMed; (c) T. Gerfaud, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2009, 48, 572 CrossRef CAS PubMed; (d) Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS PubMed; (e) F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1977 CrossRef CAS PubMed; (f) A. Faulkner and J. F. Bower, Angew. Chem. Int. Ed., 2012, 51, 1675 CrossRef CAS PubMed; (g) A. Faulkner, J. S. Scott and J. F. Bower, Chem. Commun., 2013, 49, 1521 RSC; (h) C. Chen, L. Hou, M. Cheng, J. Su and X. Tong, Angew. Chem. Int. Ed., 2015, 54, 1 CrossRef PubMed.
- (a) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC; (b) H. Huang, X. Ji, X. Tang, M. Zhang, X. Li and H. Jiang, Org. Lett., 2013, 15, 6254 CrossRef CAS PubMed; (c) X. Tang, L. Huang, Y. Xu, J. Yang, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4205 CrossRef CAS PubMed; (d) X. Tang, L. Huang, J. Yang, Y. Xu, W. Wu and H. Jiang, Chem. Commun., 2014, 50, 14793 RSC.
- (a) W. Wu and H. Jiang, Acc. Chem. Res., 2012, 45, 1736 CrossRef CAS PubMed; (b) Y. Wen, L. Huang, H. Jiang and H. Chen, J. Org. Chem., 2012, 77, 2029 CrossRef CAS PubMed; (c) H. Chen, L. Huang, W. Fu, X. Liu and H. Jiang, Chem. Eur. J., 2012, 18, 10497 CrossRef CAS PubMed; (d) L. Huang, Q. Wang, J. Qi, X. Wu, K. Huang and H. Jiang, Chem. Sci., 2013, 4, 2665 RSC; (e) L. Huang, J. Qi, X. Wu, K. Huang and H. Jiang, Org. Lett., 2013, 15, 2330 CrossRef CAS PubMed; (f) Q. Wang, L. Huang, X. Wu and H. Jiang, Org. Lett., 2013, 15, 5940 CrossRef CAS PubMed.
- (a) M. Mahyari, M. S. Laeini and A. Shaabani, Chem. Commun., 2014, 50, 7855 RSC; (b) Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D. S. Su, J. Wang, X. Bao and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 2109 CrossRef CAS PubMed; (c) G. Urgoitia, R. S. Martin, M. T. Herrero and E. Domínguez, Green Chem., 2011, 13, 2161 RSC; (d) X. Zhang, X. Ji, S. Jiang, L. Liu, B. L. Weeks and Z. Zhang, Green Chem., 2011, 13, 1891 RSC; (e) X. Feng, J.-J. Wang, Z. Xun, Z.-B. Huang and D.-Q. Shi, J. Org. Chem., 2015, 80, 1025 CrossRef CAS PubMed.
- (a) C. Ferreri, C. Costantino, C. Chatgilialoglu, R. Boukherroub and G. Manuel, J. Organometallic Chem., 1998, 554, 135 CrossRef CAS; (b) H. Wang, L. Li, X.-F. Bai, J.-Y. Shang, K.-F. Yang and L.-W. Xu, Adv. Synth. Catal., 2013, 355, 341 CAS; (c) H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046 CrossRef CAS PubMed; (d) H. Hikawa, N. Matsuda, H. Suzuki, Y. Yokoyama and I. Azumaya, Adv. Synth. Catal., 2013, 355, 2308 CrossRef CAS PubMed.
- (a) A. N. Campbel, P. B. White, I. A. Guzei and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 15116 CrossRef PubMed; (b) M. A. Bigi and M. C. White, J. Am. Chem. Soc., 2013, 135, 7831 CrossRef CAS PubMed; (c) A. Sharma and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 17983 CrossRef CAS PubMed; (d) T. Diao, T. J. Wadzinski and S. S. Stahl, Chem. Sci., 2012, 3, 887 RSC; (e) W. Gao, Z. He, Y. Qian, J. Zhao and Y. Huang, Chem. Sci., 2012, 3, 883 RSC.
- See the ESI† for the details of the control experiments.
- K. Okamoto, T. Oda, S. Kohigashi and K. Ohe, Angew. Chem., Int. Ed., 2011, 50, 11470 CrossRef CAS PubMed.
- (a) Y. Li, Z. Yu and S. Wu, J. Org. Chem., 2008, 73, 5647 CrossRef CAS PubMed; (b) M. Zheng, L. Huang, W. Wu and H. Jiang, Org. Lett., 2013, 15, 1838 CrossRef CAS PubMed; (c) A. J. Mota and A. Dedieu, J. Org. Chem., 2007, 72, 9669 CrossRef CAS PubMed; (d) T. Piou, L. Neuville and J. Zhu, Angew. Chem. Int. Ed., 2012, 51, 11561 CrossRef CAS PubMed.
- (a) R. Neumann and M. Lissel, J. Org. Chem., 1989, 54, 4607 CrossRef CAS; (b) R. Yan, J. Luo, C. Wang, C. Ma, G. Huang and Y. Liang, J. Org. Chem., 2010, 75, 5395 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds, copies of 1H and 13C NMR spectra for selected compounds. See DOI: 10.1039/c5cc06958k |
| This journal is © The Royal Society of Chemistry 2016 |