Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spatio-temporal control of cellular uptake achieved by photoswitchable cell-penetrating peptides†
Andreas
Prestel
a and
Heiko M.
Möller
*ab
aDepartment of Chemistry and Konstanz Research School Chemical Biology, University of Konstanz, 78464 Konstanz, Germany
bInstitute of Chemistry/Analytical Chemistry, University of Potsdam, 14476 Potsdam, Germany. E-mail: heiko.moeller@uni-potsdam.de
First published on 4th November 2015
Abstract
The selective uptake of compounds into specific cells of interest is a major objective in cell biology and drug delivery. By incorporation of a novel, thermostable azobenzene moiety we generated peptides that can be switched optically between an inactive state and an active, cell-penetrating state with excellent spatio-temporal control.
Cell-penetrating peptides (CPPs) are mostly polycationic and/or amphiphilic peptides with the ability to cross the hydrophobic cellular membrane.1–3 The exact mechanism of cellular uptake is still a subject of scientific discourse and there are controversial results, indicating different mechanisms, depending on the type of CPP and experimental conditions.4–6 Nevertheless, these vectors are widely used to deliver various cargos into living cells, including small molecules, macromolecules like proteins or oligonucleotides, as well as nanoparticles and liposomes.7–9 Since these peptides enter cells rather unspecifically, much effort has been made towards a selective uptake into specific cells of interest. To this end, the peptides remain in an inactive state until exposed to an external trigger like heat,11 pH12 or light-induced deprotection.13 Prominent members in the group of cationic CPPs are oligoarginines (Arg8, Arg9), which are commonly used for membrane translocation.14,15 It has been shown that their cellular uptake can be inhibited by masking the positively charged guanidinium groups of arginines using polyanionic structures like heparin.16 This was used to design activatable CPPs (ACPPs), in which the oligoarginine is linked to a polyanionic peptide via a cleavable turn-structure.17 Upon scission of the linker by specific proteases or other external factors, the polyanions dissociate and the polycationic peptides are released to deliver their cargo into the cells.17–19 These ACPP allow for controlling cellular uptake in temporal fashion. However, the spatial control is rather limited because of the diffusion of the activating reagent. Furthermore, activation of ACPPs is one-way and there is no handle to deactivate these structures.
An interesting possibility to control the function of biomolecules reversibly is the incorporation of azobenzene (AB) moieties.20–23 AB can adopt two distinct configurations of its central N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-bond (cis and trans), which can be interconverted by irradiation at appropriate wavelengths24 and the photochemical properties can be tuned by altering the substitution pattern.25 In the dark, the energetically favoured trans-AB is almost exclusively formed and it adopts a nearly planar conformation. Upon irradiation it can be converted into the cis-AB with a twisted arrangement of its benzene moieties. A photostationary state (PSS) is reached where, depending on the wavelength, either the cis-AB (λ ≈ 360 nm) or the trans-AB (λ ≈ 440 nm) is the predominant form.26 To reversibly control the function of peptides with ABs, two major principles have been reported: either cyclization via side chains27 or incorporation into the peptide backbone.28 In the latter case the twisted cis-AB is mostly used to mimic a native β-turn, while the linear, extended trans-AB prevents β-turn formation.29
N-bond (cis and trans), which can be interconverted by irradiation at appropriate wavelengths24 and the photochemical properties can be tuned by altering the substitution pattern.25 In the dark, the energetically favoured trans-AB is almost exclusively formed and it adopts a nearly planar conformation. Upon irradiation it can be converted into the cis-AB with a twisted arrangement of its benzene moieties. A photostationary state (PSS) is reached where, depending on the wavelength, either the cis-AB (λ ≈ 360 nm) or the trans-AB (λ ≈ 440 nm) is the predominant form.26 To reversibly control the function of peptides with ABs, two major principles have been reported: either cyclization via side chains27 or incorporation into the peptide backbone.28 In the latter case the twisted cis-AB is mostly used to mimic a native β-turn, while the linear, extended trans-AB prevents β-turn formation.29
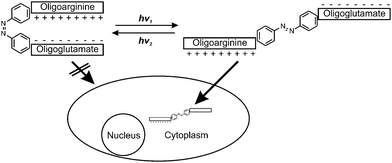
Here, we describe the synthesis of a photoswitchable CPP (PCPP) comprised of a cell-penetrating oligoarginine linked to an inhibitory oligoglutamate by an AB moiety, which is integrated into the peptide backbone. In the cis-form, the AB adopts a turn-like structure and allows for an efficient pairing of the two oppositely charged peptide sequences, whereas the extended trans-form is supposed to disrupt this pairing, release the oligoarginine and, consequently, induce cellular uptake (Scheme 1). In order to slow down thermal cis-to-trans isomerization, methyl substituents in ortho-position were introduced.30,31 To maximize the spatial rearrangement and reduce any internal flexibility, an AB was chosen that yields very rigid aromatic amides in para position after incorporation into the peptide backbone.
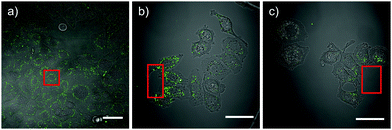
The synthesis of the AB-moiety (2) is outlined in Scheme 2a. To avoid the difficult coupling of the aromatic amine during solid phase peptide synthesis (SPPS), the first glutamate residue was attached in solution to yield compound 3, which could be coupled quantitatively in the next step. To monitor cellular uptake, the N-terminus of the peptides was modified with a rhodamine dye (4-OH), which was prepared from rhodamine B as previously described by Nguyen et al.10 The peptides were synthesized on solid support and purification was achieved via RP-HPLC (for experimental details see ESI†). Three different peptides (E6R9, E8R8 and E9R9; see Scheme 2c) were synthesized based on the design by Jiang et al.17 to investigate the influence of chain length on cellular uptake and other biophysical properties like solubility. The cis-peptides were generated by irradiation at 366 nm. After about 5 minutes a photostationary state was established, with 30–80% of the peptide converted, depending on peptide sequence and experimental conditions (solvent, pH). Irradiation at 438 nm or 495 nm reversed the isomerization and the initial state of almost 100% trans-peptide was recovered (Fig. 1a). However, the cis-peptides could be isolated by RP-HPLC using shallow gradients. In Fig. 1b consecutive UV/Vis-spectra of AcE6R9 are displayed, illustrating the thermal cis-to-trans relaxation. It is evident that the cis-peptide obtained after RP-HPLC is quite pure (>95%) and that the thermal isomerization is sufficiently slow, with a half-life of about 30 hours at 22 °C. This photochemical behaviour is representative for all peptides and conditions used in this study (for details see Fig. S2, ESI†). That the cis-peptide indeed adopts a turn-like structure could be shown by NMR spectroscopy revealing NOE contacts between side chain HN groups of Arg and Hβ and Hγ of Glu (Fig. S3, ESI†). The cellular uptake of the two isomers was investigated by confocal microscopy. In Fig. 2 the uptake of cis- and trans-flE9R9 into HeLa-cells is visualized. After 40, 90 and 135 minutes the cis-form was only taken up marginally (Fig. 2a, c and e), while the trans-form accumulated rapidly in the cytoplasm of the cells (Fig. 2f, Fig. S4 and S5, ESI†). To convert the cis-into the activated trans-peptide, a region of interest was irradiated at 488 nm with high laser intensity (240 μW; ∼0.4 ms μm−2) under the confocal microscope. During the following incubation period, the peptide was taken up by the cells at comparable levels as achieved by direct addition of the trans-peptide (Fig. 2b and d; supplementary movies, ESI†). Using the spatially restricted confocal laser beam, selected areas on the same dish could be activated while distant areas were unaffected. To quantify the uptake, the incubated cells were additionally analyzed by flow cytometry. While the trans-form of all 3 investigated peptides entered HeLa-cells very efficiently, the respective cis-peptide was taken up to a much lesser extent and inhibition was most effective for cis-flE9R9 (Fig. 2g, Fig. S6, ESI†). This light triggered translocation of the peptides could also be shown for 4 other cell lines (HCT116, BSC1, MCF7 and RPE1), documenting the broad applicability of the PCPPs (Fig. S5, ESI†). In all cases, the activated peptides show an inhomogeneous distribution in the cytoplasm of the cells (Fig. S4, ESI†). It should be noted here that a significant portion of the activated CPP might not be internalized but enriched at the plasma membrane.32 These membrane-bound CPPs might remain undetected due to fluorescence quenching.
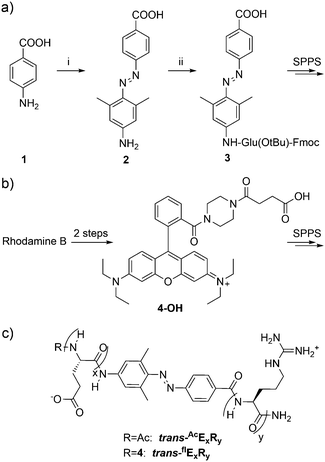 | ||
| Scheme 2 (a) Synthesis of the AB moiety. Reagents and conditions: (i) NaNO2, HClaq., 0 °C, 30 min, then 3,5-dimethylaniline, 0 °C, 2 h, 70%; (ii) Fmoc-Glu(OtBu)-OH, HATU, HOAt, DIPEA, NMP, 23 °C, 14 h. (b) Compound 4-OH was used for fluorescent labelling of the peptides and synthesized according to Nguyen et al. from Rhodamine B in 2 steps.10 (c) Structure of the PCPPs used in this study. The nomenclature of the peptides was defined as follows: the configuration of the AB moiety is denoted by the prefix ‘cis’ or ‘trans’. The fluorescently labelled peptides are indicated by a superscripted ‘fl’, the acetylated peptides by ‘Ac’. The number of consecutive glutamate and arginine residues is specified by ExRy, Three different chain lengths were investigated: x = 6 y = 9; x = y = 8 and x = y = 9. | ||
 | ||
| Fig. 1 (a) Photoswitching properties of AcE6R9 in water at 22 °C. In the dark, the trans-form of AB gives rise to a strong absorption band at 355 nm (red curve). Upon irradiation at 366 nm, A355 drops and a PSS is reached after about 5 minutes (shaded purple curves). This effect is reversed when irradiating at 438 nm (dashed blue curve on top of the red curve). (b) Thermal cis-to-trans isomerization of AcE6R9 monitored by consecutive UV/Vis-spectra. The black spectrum is obtained directly after elution from RP-HPLC and corresponds to almost pure cis-form (95.6% cis-form, see also Fig. S1 of the ESI†). The half-life of thermal isomerization is about 30 h (21% MeCN; 0.1% TFA in water, 22 °C). | ||
The intracellular distribution is indicative of a vesicular uptake mode in accordance to many reports of substituted oligoarginines.33,34 This reduces the bioavailability of the delivered compound but several endosomal escape or cleavage mechanisms are described in literature.35,36
When irradiated with a single beam of high laser intensity, the spatial resolution is limited, because peptides in a large volume above the confocal plane are also activated and can diffuse quickly to regions several 100 μm away (Fig. 3a). However, by applying strongly reduced laser intensity (1 to 1.5 μW) in a repeated fashion we were able to achieve spatially restricted uptake into a few (1–5) selected cells, while neighboring cells were only barely affected (Fig. 3b and c).
Using an azobenzene building block offers the possibility to not only photo-activate cellular uptake but also to switch the CPP back into the inactive state. Indeed, the acetylated peptide AcE9R9 could be switched back to the inactive, cis-form with 80% yield (Fig. S1, ESI†) by irradiation with UV-light (∼360 nm). Similar switching yields should be achievable with many different cargos. However, when attaching a fluorescent dye it is known that the trans-to-cis conversion yield is significantly reduced, in case of flE9R9 to ∼40% (compare RP-HPLC runs in Fig. S1, ESI†) This phenomenon was previously described for other peptides containing an AB photoswitch and different fluorophores, but the exact mechanisms for the shift of the equilibrium at the photostationary state are not known.37
When using caged compounds or photodeprotection there is at least a risk of toxicity or unwanted side effects caused by the released caging groups. In our case, we are, first, able to prevent uptake in areas that have not been irradiated, and, second, there is no release of a caging group. No toxic effect was observable using the fluorescent peptides at low micromolar concentrations in cell culture. To further evaluate the toxicity, an alamar blue® assay was performed, but no impact on cell proliferation was detected even after prolonged exposure at concentrations up to 40 μM (Fig. S7, ESI†). To our knowledge, this is the first report of a photoswitchable cell penetrating peptide and this vector could be used to deliver various conjugated cargos to selected cells with excellent spatial and temporal resolution.
We would like to thank Prof. Dr T. U. Mayer and A. Brendel (cell culture); Prof. Dr M. Groettrup and Dr M. Basler (flow cytometry) as well as Prof. Dr E. May and D. Hermann (confocal microscopy at the Bioimaging Center of the University of Konstanz) for their expertise, and infrastructural support. Financial support through the University of Konstanz is gratefully acknowledged. We would like to thank Prof. Dr V. Wittmann for critically reading the manuscript.
Notes and references
- A. D. Frankel and C. O. Pabo, Cell, 1988, 55, 1189–1193 CrossRef CAS PubMed.
- J. P. Richard, K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gait, L. V. Chernomordik and B. Lebleu, J. Biol. Chem., 2003, 278, 585–590 CrossRef CAS PubMed.
- M. Lindgren, M. Hällbrink, A. Prochiantz and Ü. Langel, Trends Pharmacol. Sci., 2000, 21, 99–103 CrossRef CAS PubMed.
- F. Madani, S. Lindberg, U. Langel, S. Futaki and A. Graslund, J. Biophys., 2011, 2011, 414729 Search PubMed.
- F. Milletti, Drug Discovery Today, 2012, 17, 850–860 CrossRef CAS PubMed.
- S. Stalmans, E. Wynendaele, N. Bracke, B. Gevaert, M. D’Hondt, K. Peremans, C. Burvenich and B. De Spiegeleer, PLoS One, 2013, 8, e71752 CAS.
- S. T. Henriques, M. N. Melo and M. A. Castanho, Biochem. J., 2006, 399, 1–7 CrossRef CAS PubMed.
- F. Heitz, M. C. Morris and G. Divita, Br. J. Pharmacol., 2009, 157, 195–206 CrossRef CAS PubMed.
- N. Nischan, H. D. Herce, F. Natale, N. Bohlke, N. Budisa, M. C. Cardoso and C. P. R. Hackenberger, Angew. Chem., Int. Ed., 2015, 54, 1950–1953 CrossRef CAS PubMed.
- T. Nguyen and M. B. Francis, Org. Lett., 2003, 5, 3245–3248 CrossRef CAS PubMed.
- R. L. Bartlett, 2nd, S. Sharma and A. Panitch, Nanomedicine, 2013, 9, 419–427 CrossRef PubMed.
- E. Jin, B. Zhang, X. Sun, Z. Zhou, X. Ma, Q. Sun, J. Tang, Y. Shen, E. Van Kirk, W. J. Murdoch and M. Radosz, J. Am. Chem. Soc., 2013, 135, 933–940 CrossRef CAS PubMed.
- Y. Shamay, L. Adar, G. Ashkenasy and A. David, Biomaterials, 2011, 32, 1377–1386 CrossRef CAS PubMed.
- S. Futaki, T. Suzuki, W. Ohashi, T. Yagami, S. Tanaka, K. Ueda and Y. Sugiura, J. Biol. Chem., 2001, 276, 5836–5840 CrossRef CAS PubMed.
- D. M. Copolovici, K. Langel, E. Eriste and U. Langel, ACS Nano, 2014, 8, 1972–1994 CrossRef CAS PubMed.
- S. M. Fuchs and R. T. Raines, Biochemistry, 2004, 43, 2438–2444 CrossRef CAS PubMed.
- T. Jiang, E. S. Olson, Q. T. Nguyen, M. Roy, P. A. Jennings and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17867–17872 CrossRef CAS PubMed.
- R. Weinstain, E. N. Savariar, C. N. Felsen and R. Y. Tsien, J. Am. Chem. Soc., 2014, 136, 874–877 CrossRef CAS PubMed.
- M. Whitney, E. N. Savariar, B. Friedman, R. A. Levin, J. L. Crisp, H. L. Glasgow, R. Lefkowitz, S. R. Adams, P. Steinbach, N. Nashi, Q. T. Nguyen and R. Y. Tsien, Angew. Chem., Int. Ed., 2013, 52, 325–330 CrossRef CAS PubMed.
- A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC.
- M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2014, 53, 3264–3267 CrossRef PubMed.
- T. Fehrentz, M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2011, 50, 12156–12182 CrossRef CAS PubMed.
- L. Nevola, A. Martín-Quirós, K. Eckelt, N. Camarero, S. Tosi, A. Llobet, E. Giralt and P. Gorostiza, Angew. Chem., 2013, 125, 7858–7862 CrossRef.
- G. S. Hartley, Nature, 1937, 140, 281 CrossRef CAS.
- O. Sadovski, A. A. Beharry, F. Zhang and G. A. Woolley, Angew. Chem., Int. Ed., 2009, 48, 1484–1486 CrossRef CAS PubMed.
- W. R. Brode, J. H. Gould and G. M. Wyman, J. Am. Chem. Soc., 1952, 74, 4641–4646 CrossRef CAS.
- J. R. Kumita, O. S. Smart and G. A. Woolley, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3803–3808 CrossRef CAS.
- A. Cattani-Scholz, C. Renner, C. Cabrele, R. Behrendt, D. Oesterhelt and L. Moroder, Angew. Chem., Int. Ed., 2002, 41, 289–292 CrossRef CAS.
- C. Hoppmann, S. Seedorff, A. Richter, H. Fabian, P. Schmieder, K. Rück-Braun and M. Beyermann, Angew. Chem., 2009, 121, 6763–6766 CrossRef.
- H. Nishioka, X. Liang and H. Asanuma, Chem. – Eur. J., 2010, 16, 2054–2062 CrossRef CAS PubMed.
- M. R. Han, D. Hashizume and M. Hara, New J. Chem., 2007, 31, 1746–1750 RSC.
- A. Walrant, I. Correia, C.-Y. Jiao, O. Lequin, E. H. Bent, N. Goasdoué, C. Lacombe, G. Chassaing, S. Sagan and I. D. Alves, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 382–393 CrossRef CAS PubMed.
- T. Tsumuraya and M. Matsushita, PLoS One, 2014, 9, e86639 Search PubMed.
- S. M. Fuchs and R. T. Raines, Biochem. J., 2004, 43, 2438–2444 CrossRef CAS PubMed.
- H. Fuchs, C. Bachran and D. Flavell, Antibodies, 2013, 2, 209–235 CrossRef CAS.
- A. K. Varkouhi, M. Scholte, G. Storm and H. J. Haisma, J. Controlled Release, 2011, 151, 220–228 CrossRef CAS PubMed.
- A. A. Beharry, L. Wong, V. Tropepe and G. A. Woolley, Angew. Chem., Int. Ed., 2011, 50, 1325–1327 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc06848g |
| This journal is © The Royal Society of Chemistry 2016 |