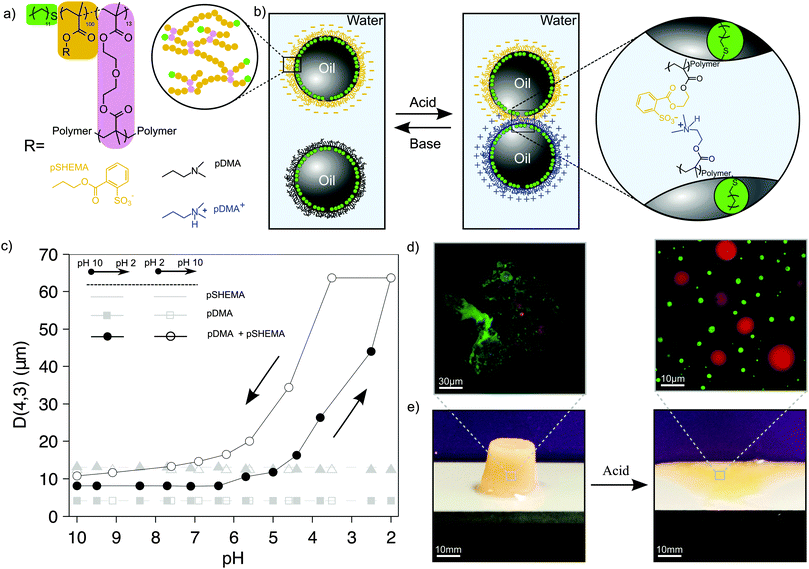
Reversible assembly of pH responsive branched copolymer-stabilised emulsion via electrostatic forces†‡
Anthony L. B.
Maçon§
 *,
Saif Ur
Rehman§
,
Robert V.
Bell
and
Jonathan V. M.
Weaver¶
*,
Saif Ur
Rehman§
,
Robert V.
Bell
and
Jonathan V. M.
Weaver¶
Imperial College London, Materials Department, Exhibition Road, SW7 2AZ, London, UK. E-mail: maconanthony@icloud.com; Tel: +44 (0)7 598 776 546
First published on 22nd October 2015
Abstract
The judicious compositional and structural design of a branched co-polymeric surfactant allows for the production of highly stable oil in water emulsion droplets with reversible electrostatic aggregation behaviour.
Many natural and biological entities or processes acquire their unique properties through selective non-covalent interactions, as found in proteins, viruses or extracellular matrices.1–4 Being able to understand and synthetically replicate these molecular interactions has become an increasingly active research endeavour. One approach is to use highly stable emulsion droplets as model surface-functionalised materials where molecular-recognition can lead to inter-droplets assembly to give larger, more robust, aggregated structures.5
In previous work, architecturally and compositionally well defined branched copolymer surfactants (BCSs) were synthesised and used to produce highly stable oil-in-water emulsions.6,7 With careful design of the composition, functionalities present at this interface can be utilised to allow triggerable interaction between droplets.8 Thus, pH induced reversible emulsion assembly was achieved when preparing emulsions using BCS composed of poly(ethyleneglycol) methacrylate (PEGMA) and methacrylic acid (MA) at a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between EG to MA residues.6 At a neutral/basic pH, MA residues provides electrostatic stabilisation, this coupled with the steric stabilisation by EG chains in the PEGMA give rise to discrete, well dispersed, emulsion droplets. Upon lowering the pH of the aqueous phase, protonation of the MA moiety (pKa ≈ 5) induces intradroplet hydrogen-bonds with the EG repeating unit. If the droplet concentration is high enough, interdroplet hydrogen-bonds forms, giving the faculty of a disperse emulsion to assemble, falling under the precept of “Emulsion engineering”.9–11
1 between EG to MA residues.6 At a neutral/basic pH, MA residues provides electrostatic stabilisation, this coupled with the steric stabilisation by EG chains in the PEGMA give rise to discrete, well dispersed, emulsion droplets. Upon lowering the pH of the aqueous phase, protonation of the MA moiety (pKa ≈ 5) induces intradroplet hydrogen-bonds with the EG repeating unit. If the droplet concentration is high enough, interdroplet hydrogen-bonds forms, giving the faculty of a disperse emulsion to assemble, falling under the precept of “Emulsion engineering”.9–11
Herein, we demonstrate that by modifying the chemical composition of these BCSs, interdroplet assembly can be driven by electrostatic forces. A set of two amphiphilic branched co-polymers were synthesised using a thiol-regulated free radical polymerisation and based on the optimisation studies previously conducted.12 A cationic pH responsive and a permanent negatively charged BCSs were synthesised using 2-(dimethylamino)ethyl methacrylate (DMA) and 2-(sulfobenzoic acid)ethyl methacrylate (SHEMA) as a main repeating unit, respectively (Fig. 1). The latter was obtained from the esterification of poly(2-hydroxyethyl methacrylate) with 2-sulfobenzoic acid cyclic anhydride. Both surfactants were designed on the same principle: (i) 1-dodecanethiol (DDT) was used as a chain transfer agent, giving chain ends that have the ability to strongly adsorb at the oil interface of an emulsion droplet. (ii) This physical adsorption was reinforced by branching out these polymers using di(ethyleneglycol) dimethacrylate (DEGDMA). Monomolecular compact structures were obtained as shown by the molecular weight (Mn), Mark–Houwink-α (MH-α) and dynamic light scattering (Dh) values given in Table 1 (chromatogram and spectra given in the ESI‡), allowing the surfactants to anchor the hydrophobic phase from multiple sites and thereby intensively increasing their efficiency as compared to their linear homologues.13 The actual compositions of the branched co-polymers synthesised in this work were in good agreement with the targeted composition (Table 1). This was confirmed by 1H NMR (Fig. 1).
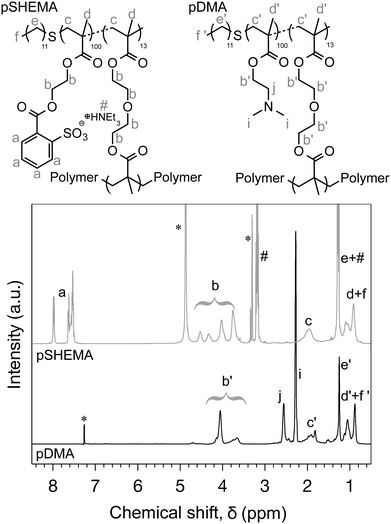 | ||
| Fig. 1 1H NMR spectra of pDMA in CDCl3, and pSHEMA in MeOD, and their corresponding chemical structure. * represent deuterated solvents. # represents triethylamine. | ||
| Sample | Feed ratioa | Actual ratiob | M n (g mol−1) | PDIc | MH-αc | D h (nm) | pKae | Emulsion | |
|---|---|---|---|---|---|---|---|---|---|
| X/DEGDMA/DDT | X/DEGDMA/DDT | D(4,3)g (μm) | pKaf | ||||||
| a Polymer targeted composition where X is the functional monomer: 2-(dimethylamino)ethyl methacrylate for pDMA, 2-hydroxyethyl methacrylate for pHEMA and 2-(sulfobenzoic acid)ethyl methacrylate for pSHEMA. pSHEMA was obtained by esterification of pHEMA with 2-sulfobenzoic acid cyclic anhydride. b Determined by 1H NMR. c Determined by triple detection GPC. PDI represent the polydispersity index, ratio of the mass average molecular weight Mw and the number average molecular weight Mn. MH-α is the Mark–Houwin-α coefficient. d Obtained from polymer solubilised in water at pH 2 by dynamic light scattering. e Obtained by titration on free pDMA solubilised in water. f Obtained by titration on the emulsion droplet. g Determined by laser diffraction of the emulsion droplets in water at pH 2. | |||||||||
| pDMA | 100/13/15 | 100/13/13 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654 |
1.54 | 0.33 | 11 | 6.7 ± 0.1 | 5.4 | 6.5 ± 0.1 |
| pHEMA | 100/13/15 | 100/16/16 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
1.63 | 0.32 | 12 | — | — | — |
| pSHEMA | 100/13/15 | 100/16/16 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
1.62 | 0.38 | 18 | — | 13.6 | — |
Under acidic conditions and below its pKa, poly(DMA) is known to bear a positive charge due to the protonation of its tertiary amine, which can be reversibly removed upon increase of the pH above its pKa. The pH transition range, or buffering capacity, between the protonated and deprotonated amine can be evaluated by titration (titration curve available in ESI‡) and considerably increased upon branching using DEGDMA as a co-monomer, going from pH 4 to pH 8 with a corresponding pKa of 7.4 for linear poly(DMA)14 to pH 4 to 10.5 with a corresponding pKa of 6.7 when branched. The increase in buffering capacity and the decrease of the pKa is a direct consequence of the steric constraints imparted on the branched polymers compared to linear polymer.12 Thus, pDMA was soluble from pH 2 to pH 10, the working pH used in the emulsion characterisation, where linear poly(2-(dimethylamino)ethyl methacrylate) were reported to precipitate above pH 8. In addition, the surface charge of pDMA merely varied from pH 2 to 10, ζpDMA = 22 mV, corroborating the titration data.
2-Hydroxyethyl methacrylate (HEMA) was polymerised and subsequently functionalised by esterification of its alcohol residue with 2-sulfobenzoic acid cyclic anhydride (SBA) with a yield of 85%.15 Through the conversion of hydroxyls to sulfonates, the ζ-potential of the BCS fell from −4 mV to −30 mV, independently of the pH (pH ∈ [2;10], figure in the ESI‡). Thus, this means that over this range of pH, pSHEMA bears a permanent negative charge. Interestingly, the functionalised poly(HEMA) (pSHEMA) saw an increase in its MH-α from 0.32 to 0.38 indicating that upon esterification, pSHEMA dilated its structure inducing a better affinity for aqueous solvent. This increase could also be explained by a structural rearrangement of the BCS to accommodate the steric effect induced by introduction of the aromatic ring of SBA.
The efficiency of the amphiphilic branched co-polymers was accessed by measuring the interfacial surface tension (IST) of the water–BCS–dodecane system using a drop-volume tensiometer (table and figure in ESI‡). The tension between the two non-miscible liquid decreased drastically when any of a BCS was present in the aqueous phase over a wide range of pH. For example, the IST went from 40.2 mN m−1 when the aqueous solution was surfactant-free, to 8.2 mN m−1 when pDMA or pSHEMA was present in solution at pH 2. These values were remarkably lower than commercially available surfactants using similar experimental conditions (ISTSDS = 33.2 mN m−1; ISTBrij58 = 18.5 mN m−1; ISTCTAB = 34.6 mN m−1).16 This is highlighting that stronger droplet adhesion was afforded by the multiple hydrophobic chain ends of BCSs as compared to the single hydrophobic anchorage of the standard surfactants.
Two emulsions were prepared in separate vials, one stabilised by pSHEMA and the other by pDMA. In both cases, 2 w/v% of BCS was dissolved in distilled water (pH adjusted to 2 by the addition of 0.1 M HCl). An equal volume of n-dodecane was added and the solution homogenised for 2 min at 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The emulsions were left overnight to equilibrate before any further analysis was performed. The emulsion droplets creamed due to the lower oil density (Fig. S6, ESI‡). Both emulsions appeared to be highly stable with no demulsification was observed over 2 months with a similar oil volume fraction, Φoil ≈ 0.75. The volume average diameter of the droplets (D(4,3)) was characterised by laser diffraction (Fig. 2). Although, the two BCSs were lowering the interfacial surface tension to the same extent, emulsion droplets formed with pDMA were found to be smaller, D(4,3)pDMA = 5.4 μm, than the droplets by pSHEMA, D(4,3)pSHEMA = 13.6 μm, which however stood constant overtime in mode and span. The larger size of pSHEMA stabilised droplets might be due to the steric effect caused by SBA residues or the electrostatic repulsion between adjacent BCSs. However, the exact nature of this difference in size remains unclear. Both systems were found to be highly stable upon increase of the pH from 2 to 10 as shown in Fig. 2c. Interestingly, the surface charge of the droplets were similar to the values obtained for the free polymers in solution. This is a good indication that the SBA and dimethylamino residues were preferentially located in the aqueous phase providing specific surface functionality to these micro-scale objects. However, the pKa of the droplets stabilised with pDMA was found to be lower than when pDMA was free in solution (Table 1). This may suggest that the amine residues were dynamically moving back and forth from the aqueous phase to the oil phase without compromising the stability of the emulsion droplets.
000 rpm. The emulsions were left overnight to equilibrate before any further analysis was performed. The emulsion droplets creamed due to the lower oil density (Fig. S6, ESI‡). Both emulsions appeared to be highly stable with no demulsification was observed over 2 months with a similar oil volume fraction, Φoil ≈ 0.75. The volume average diameter of the droplets (D(4,3)) was characterised by laser diffraction (Fig. 2). Although, the two BCSs were lowering the interfacial surface tension to the same extent, emulsion droplets formed with pDMA were found to be smaller, D(4,3)pDMA = 5.4 μm, than the droplets by pSHEMA, D(4,3)pSHEMA = 13.6 μm, which however stood constant overtime in mode and span. The larger size of pSHEMA stabilised droplets might be due to the steric effect caused by SBA residues or the electrostatic repulsion between adjacent BCSs. However, the exact nature of this difference in size remains unclear. Both systems were found to be highly stable upon increase of the pH from 2 to 10 as shown in Fig. 2c. Interestingly, the surface charge of the droplets were similar to the values obtained for the free polymers in solution. This is a good indication that the SBA and dimethylamino residues were preferentially located in the aqueous phase providing specific surface functionality to these micro-scale objects. However, the pKa of the droplets stabilised with pDMA was found to be lower than when pDMA was free in solution (Table 1). This may suggest that the amine residues were dynamically moving back and forth from the aqueous phase to the oil phase without compromising the stability of the emulsion droplets.
The two emulsions were mixed at an equal volume ratio and at pH 10 where pDMA is not fully protonated. It was expected that upon decrease of the pH, i.e. full protonation of the amine residues of pDMA, the disperse emulsion droplets aggregate due to the electrostatic interaction of the positively charged pDMA and the permanently negatively charged pSHEMA as shown in the schematic presented in Fig. 2a. The aggregation phenomena of emulsion droplets (Fig. 2b) was study by laser diffraction (Fig. 2c) and confocal (Fig. 2d) imaging where two hydrophobic fluorescent dyes were dissolved in the n-dodecane oil (0.03 w/v%), prior to homogenisation. As the pH decreased, visible aggregation (D(4,3)mix > D(4,3)pSHEMA) was observed from pH 5.8, corresponding to a protonation ratio of the amine group of 83% (Fig. 2b). However, complete disassembly was reached at pH 7.6, where 26% of the amine group are still protonated. This hysteresis behaviour was assumed to be (i) a direct effect of the nature of the electrostatic forces that caused the emulsion droplets to aggregate. These forces, attracting two droplets of opposite charge, are inversely proportional to their distance, meaning that a substantial ionic strength is required to break these dynamic bonds as the droplets are in contact when assembled.17 (ii) As the aggregation took place, the diffusion of hydroxide, to break the assembly, and cations, to stabilised SBA residues, were rather limited.18 Despite the uncertainty on the mechanism, the average diameter of the mix came back to its original value. Therefore, no coalescence of the droplets occurred at low pH when aggregated, which also means that the BCSs provided enough stability to the droplet to withstand the electrostatic forces present at their surfaces and preserve their integrity. This was confirmed by light microscopy (figure in the ESI‡). At last, as seen in Fig. 2e, the emulsion assembly could be easily shaped upon aggregation and retained its shape when unmolded.
In conclusion, we are reporting for the first time the use of electrostatic forces to induce the reversible formation of engineered emulsion. Two set of BCSs were synthesised and characterised, with one bearing a permanent negative charge and the other a pH dependent positive charge, based on the protonation of the amine residue of DMA. The aggregation of the disperse emulsion droplets was shown to be reversibly switchable upon increase or decrease of the pH around the pKa of poly(DMA). Due to their compact monomolecular nature and the multiple hydrophobic anchoring sites, the BCSs were highly effective at keeping the integrity of their corresponding emulsion droplets (no coalescence or demulsification) upon aggregation and disaggregation. This novel type of interaction in engineering emulsion has the great potential to be used as a model to study natural occurring processes involving dynamic electrostatic forces.
This work is dedicated to the memory of our mentor and colleague, Dr Jonathan V. M. Weaver. The authors would like to thanks the Royal Society University (UF090640) and the Islamic Development Bank for Merit scholarship for science and technology for funding.
References
- J. K. Mouw, G. Ou and V. M. Weaver, Nat. Rev. Mol. Cell Biol., 2014, 15, 771–785 CrossRef CAS PubMed.
- T. Stylianopoulos, M.-Z. Poh, M. G. Bawendi, D. Fukumura, L. L. Munn and R. K. Jain, Biophys. J., 2010, 99, 1342–1349 CrossRef CAS PubMed.
- V. Liljeström, J. Mikkilä and M. A. Kostiainen, Nat. Commun., 2014, 5, 4445 Search PubMed.
- F. B. Sheinerman, R. Norel and B. Honig, Curr. Opin. Struct. Biol., 2000, 10, 153–159 CrossRef CAS PubMed.
- R. T. Woodward, L. Chen, D. J. Adams and J. V. M. Weaver, J. Mater. Chem., 2010, 20, 5228–5234 RSC.
- J. V. M. Weaver, S. P. Rannard and A. I. Cooper, Angew. Chem., 2009, 121, 2165–2168 CrossRef.
- R. T. Woodward, R. A. Slater, S. Higgins, S. P. Rannard, A. I. Cooper, B. J. L. Royles, P. H. Findlay and J. V. M. Weaver, Chem. Commun., 2009, 3554–3556 RSC.
- R. T. Woodward and J. V. M. Weaver, Polym. Chem., 2011, 2, 403–410 RSC.
- W. C. K. Poon, MRS Bull., 2004, 29, 96–99 CrossRef CAS.
- M. E. Leunissen, C. G. Christora, A.-P. Hynninen, C. P. Royall, A. I. Campbell, A. Imhof, M. Dijkstra, R. van Roij and A. van Blaarderen, Nature, 2005, 437, 235–240 CrossRef CAS PubMed.
- A. C. Arsenault, D. P. Puzzo, I. Manners and G. A. Ozin, Nat. Photonics, 2007, 1, 468–472 CrossRef CAS.
- J. V. M. Weaver, R. T. Williams, B. J. L. Royles, P. H. Findlay, A. I. Coopera and S. P. Rannard, Soft Matter, 2008, 4, 985–992 RSC.
- P. Chambon, L. Chen, S. Furzeland, D. Atkins, J. V. M. Weaver and D. J. Adams, Polym. Chem., 2010, 2, 941–949 RSC.
- P. van de Wetering, N. J. Zuidam, M. J. va Steenbergen, O. A. G. J. van der Houwen, W. J. M. Underberg and W. E. Hennink, Macromolecules, 1998, 31, 8063–8068 CrossRef CAS.
- C.-D. Vo, P. D. Iddon and S. P. Armes, Polymer, 2007, 48, 1193–1202 CrossRef CAS.
- S. R. Deshiikan, D. Bush, E. Eschenazi and K. D. Papadopoulos, Colloids Surf., A, 1998, 136, 130–150 CrossRef.
- M. E. Leunissen, A. van Blaaderen, A. D. Hollingsworth, M. T. Sullivan and P. M. Chaikin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2585–2590 CrossRef CAS PubMed.
- D. Topgaard, C. Malmborg and O. Söderman, J. Magn. Reson., 2002, 156, 195–201 CrossRef CAS PubMed.
Footnotes |
| † In memory of Jon Weaver. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc06636k |
| § Contributed equally. |
| ¶ Deceased. |
| This journal is © The Royal Society of Chemistry 2016 |